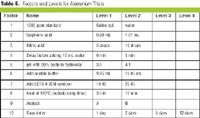
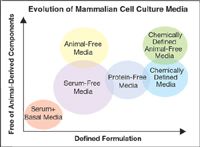
Disposable products and systems have come a long way since they first entered the small-lab market in the 1970s. Today they are available for practically every aspect of biopharmaceutical manufacturing. Disposable systems are used for filtration, clarification, purification, and separation applications used in the production of vaccines, monoclonal antibodies, and other therapies. As the use of disposable systems grows, the concept of a completely disposable manufacturing process is becoming a reality.