
No time for QbD? How to convince management to make it a priority.

No time for QbD? How to convince management to make it a priority.
Using multivariate experiments to define acceptable ranges.

There could be a serious glut of commercial scale mammalian cell culture capacity over the next five years. Then again, there could be a significant shortage. It all depends on how things develop in expression technology, the new product pipeline, and corporate strategies.

A centralized quality control strategy may be the best solution.
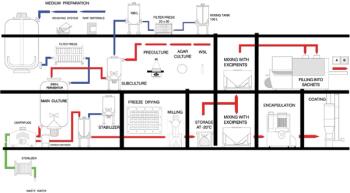
There are a number of specific characteristics to be considered when developing and manufacturing live bacterial vaccines.

The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.
Avoid manufacturing failures by effective viral inactivation.

To achieve the right balance between disposable and reuseable options, companies must consider important technical and economic factors.

There are several challenges associated with protecting patents for personalized medicines.

How to implement a risk-based approach to eliminating viruses.
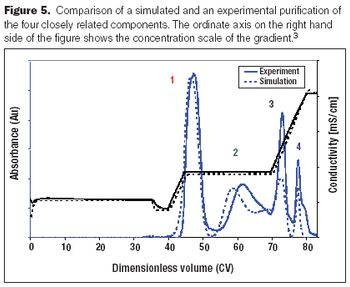
A review of some recent contributions in process chromatography.
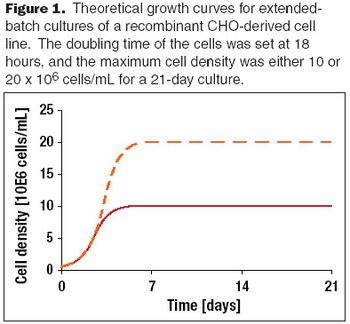
A discussion of past achievements and future expectations of recombinant protein production yields from mammalian cells.

With a variety of recombinant, animal-free, defined protein supplements such as growth factors, transferrin, and albumin entering the market, the biopharmaceutical industry now has innovative and safer alternatives to serum and other animal-derived supplements.
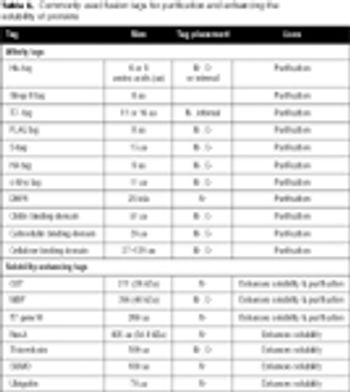
Gene fusion tags can improve the yield and solubility of many recombinant proteins. This article discusses the most popular fusion tags and the proteases used to remove them, with special reference to recently introduced technologies.
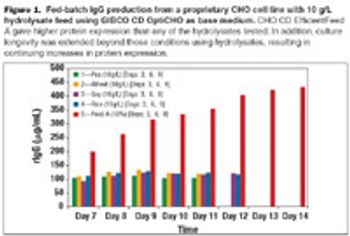
Using chemically defined feeds with CHO cell lines not only eliminates the variability associated with using plant hydrolysates, but could also improve the productivity of biopharmaceutical protein manufacture and help move therapeutic proteins into clinical trials more rapidly.
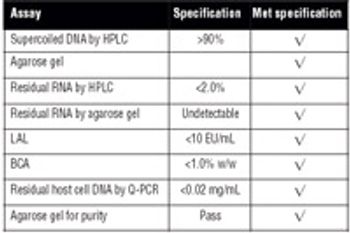
Recombinant protein and plasmid DNA production using microbial expression systems is the cornerstone of many biologics manufacturing processes. HCD methods are commonly used for these processes because of the advantages they provide.
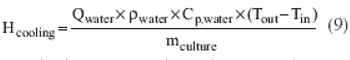
Process-modeling tools can ensure smooth tech transfer.

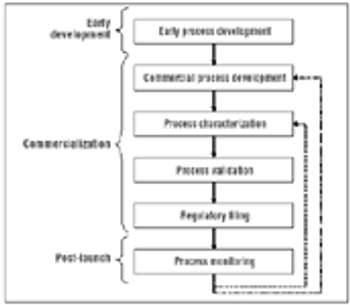
In the context of process validation, the confirmation of a belief must be checked repeatedly, throughout the product lifecycle.
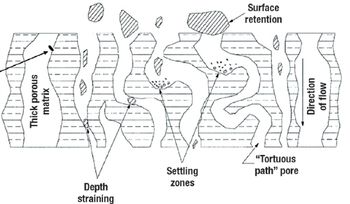
A comparison of primary harvest techniques.

Disposable technologies that mimic the conventional stainless-steel bioreactor will be most readily adopted
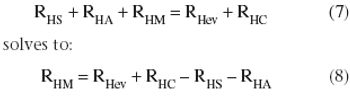
A simple method to leverage fermentation heat transfer data.
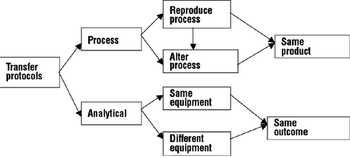
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.

Two case studies illustrate a systematic approach.

The use of disposables has changed significantly in the biopharmaceutical industry.

Chinese hamster ovary (CHO) cells are used extensively in the biopharmaceutical industry to produce recombinant proteins that require post-translation modification for full biological functionality. Optimization of culture conditions for recombinant CHO cell lines presents challenges in light of the diverse nutritional requirements observed with different clonally derived cell lines.