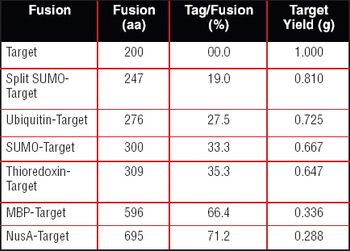
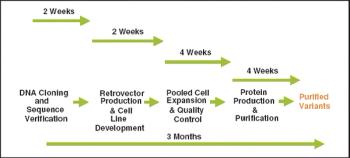
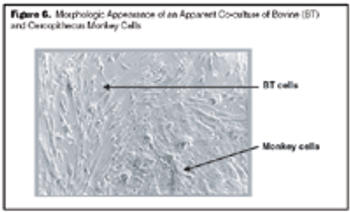
Instead of investing in new facilities, the industry should focus on improving manufacturing technology to increase yields, says Timothy Charlebois, PhD, director of cell and molecular sciences for Wyeth (Madison, NJ, www.wyeth.com). Charlebois made these remarks in his introduction to the session, "Frontiers and Economics of Mammalian Cell Expression," at the BIO 2006 convention."I've seen examples where we took a process that produced 3 grams of protein per liter, and were able to optimize it so that it produced 9.6 g/L," he said, adding that future yields are likely to be above 10 g/L.