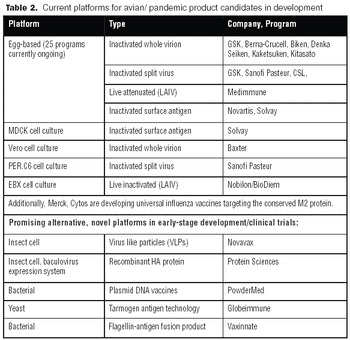
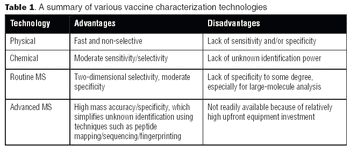
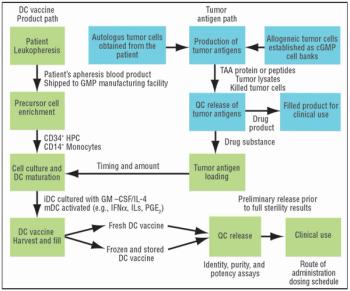
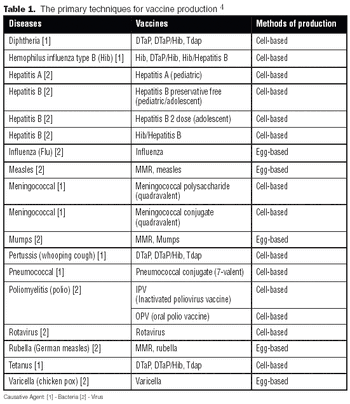
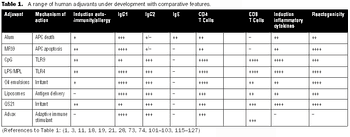
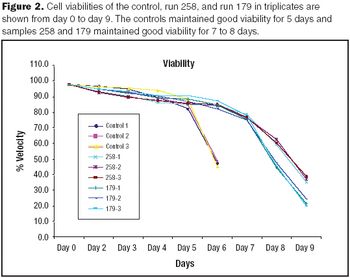
It is commonly believed that technologies in the next 10–15 years will enable sequencing an individualized human genome for less than $1,000. With innovations like these, the twenty-first century will certainly belong to biotechnology. From an industrial standpoint, the discovery of therapeutic molecules and the development of cell lines and processes to produce these molecules will be of paramount importance. This article describes various approaches that have been prevalent in the industry or are likely to be used in the future for generating cell lines with desirable traits and developing high titer cell culture processes.