The single-use ReadyToProcess WAVE 25 bioreactor system from GE Healthcare Life Sciences is a cell culture device for designed for working volumes in the 0.3 to 25 L range.

The single-use ReadyToProcess WAVE 25 bioreactor system from GE Healthcare Life Sciences is a cell culture device for designed for working volumes in the 0.3 to 25 L range.
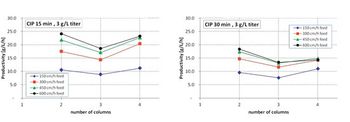
A twin-column periodic countercurrent capture process that combines an efficient sequential, countercurrent loading process with a minimal twin-column hardware configuration is described.

Industry experts discuss the implementation of QbD and PAT tools in biopharmaceutical manufacturing.

An analytical method assesses the effects of media condition on cultured antibodies.
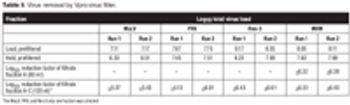
This study on a recombinant human follicle stimulating hormone demonstrates the use of virus filters to reduce the risk of contamination.

Jerold Martin considers the types of tubing available to the industry and how to make an informed selection.
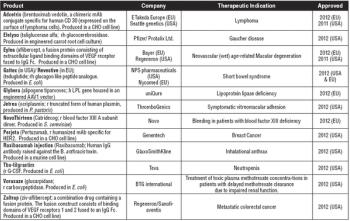
First gene therapy and plant-based expression vector products approved in 2012.

Applications of ZFN technology in biopharmaceutical cell-line engineering.

The authors describe the growth characteristics of human mesenchymal stem cells cultured in a stirred-tank bioreactor.
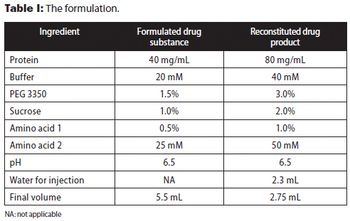
The authors present approaches used to reduce reconstitution time of a lyophilized high-concentration protein drug product.
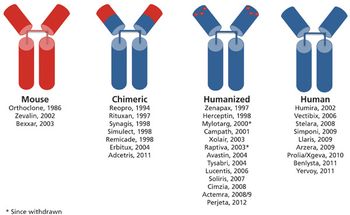
Innovative products and a range of indications drive the therapeutic antibody market.

A look at vaccine history, markets, manufacturing, and overcoming the scale-up dilemma.

Tony Hitchcock of Cobra Biologics discusses challenges posed by production of viral vectors for vaccines.

What the industry's future holds and what needs to be done to get there.
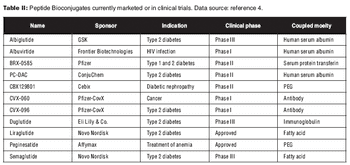
Conjugation of a biologic to a carrier molecule can solve problems in solubility and stability, but introduces its own challenges.

GE Healthcare Life Sciences' ReadyToProcess platform aims to streamline bioprocessing.
Scaling up stem-cell cultures requires careful consideration of the bioreactor design.
This month, Jerold Martin of Pall Life Sciences takes a look at protein recovery through direct-flow microporous membrane filters over the past 25 years.

The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on mAbs.

Patrick Jackson of Vindon Scientific offers key considerations for choosing an outsourced sample storage facility.

This article discusses the evaluation of a novel single-use fluidized bed centrifuge for harvesting of antibodies.

The manufacturing capacity-sharing model between Merck and MedImmune ushers in a new paradigm of "co-opetition."

A new report highlights the industry's contributions to neglected diseases and calls for further collaboration.
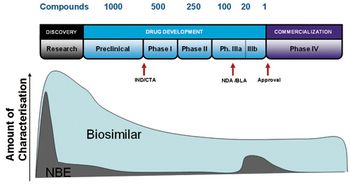
Understanding opportunities and challenges across all major phases of development.
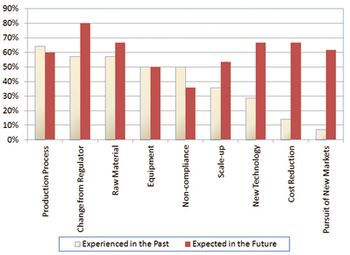
MIT survey results address product and site characteristics that statistically correlate with quality performance.