
EMD Millipore will provide process development services for Precision Biologics’ preclinical monoclonal antibody.

EMD Millipore will provide process development services for Precision Biologics’ preclinical monoclonal antibody.

They may not be glamorous, but buffers play an important role in biopharma manufacturing.
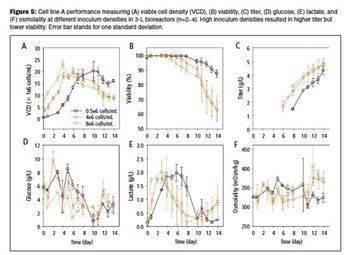
The use of commercially available media to achieve high titer in early process development is discussed.

Catalent announced that it would partner with Mitsubishi Gas Chemical Company, and its subsidiary MGC Pharma, to promote GPEx technology, a high-titer vector for stable mammalian cell lines.

Sanofi will tap into Boehringer Ingelheim’s therapeutic monoclonal antibody manufacturing capabilities.

Single-use components aid efficiency in automated personalized therapy manufacturing.

Improving efficiency, value chain, quality, and protein complexity with advanced bioprocess development.

Protein Sciences will evaluate sourcing Flublok from its Japanese licensee, UMN Pharma, which already runs a large-scale facility for the vaccine.

The Cell Therapy Catapult, a UK non-profit center for advancing cell and gene therapies, will manage the manufacturing center, which will be used for late-phase clinical trials and commercial supply.

Under an NIH contract, Paragon Bioservices will design a manufacturing process for recombinant human rhE-selectin protein.

Representatives from industry supplier companies address innovations, reliability, barriers to adoption, and the role of singIe-use bioreactors in bioprocessing.

Challenges remain for virus removal and validation.

Eppendorf's Cell Culture Consumables have an ISO class/GMP class C clean room production standard.

Fed-batch processes were scaled up from traditional bench-scale bioreactors to large-scale single-use systems.
Careful selection of downstream processing conditions is a must.

Different approaches to prepare highly concentrated feed media for fed-batch Chinese hamster ovary cell culture are evaluated.

New approaches to vaccine production are targeting rapid supply for pandemic situations and broadly effective therapeutic treatments.

Gallus BioPharmaceuticals enters a cell line optimization and manufacturing agreement with Omni Bio Pharmaceutical.

New human and plant-based expressions systems can enable faster product development and improve quality at potentially lower costs.
USP evaluates raw materials used in the chemical synthesis of peptides.
The biopharmaceutical industry is developing a new approach to controlling variability in raw materials.
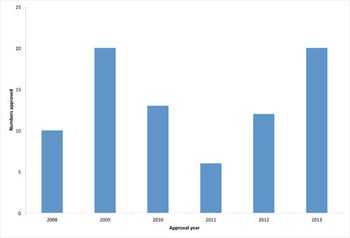
A review of the past year's trends in biopharmaceutical approvals shows an above average approval rate.

Researchers are using current understanding of the lyophilization process to predict performance on many levels during both process development and manufacturing.

CMOs may find opportunities in alternative expression services.

Key considerations for implementing single-use components or platforms when moving from research to process development.