
Steven S. Kuwahara, PhD, principal consultant at GXP BioTechnology LLC, gives an update on "Engineering the Cell-System Interface."

Steven S. Kuwahara, PhD, principal consultant at GXP BioTechnology LLC, gives an update on "Engineering the Cell-System Interface."
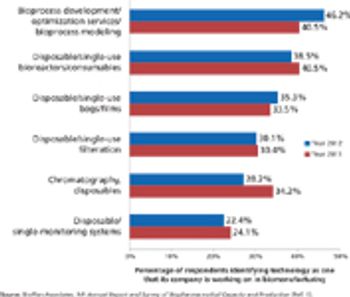
Industry wants more innovation, but can suppliers meet customers' needs?

This article contains online-exclusive supplemental material for Malik's article entitled, "Allogenic Versus Autologous Stem-Cell Therapy."
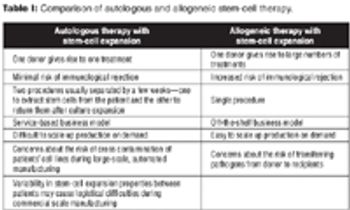
The author discusses potential manufacturing costs & challenges of allogeneic & autologous stem-cell therapy.
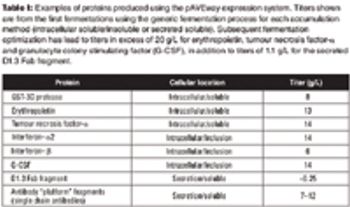
Industry experts discuss methods for optimizing protein expression in bacterial and mammalian cell lines.
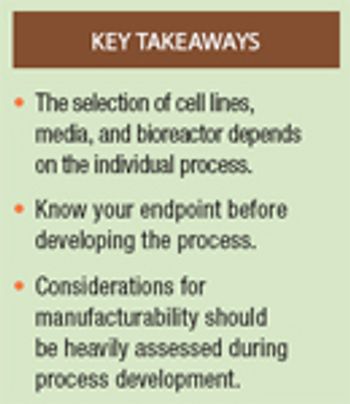
Selection of the right cell line, culture medium, and bioreactor conditions is key to setting up the upstream portion of the biopharmaceutical manufacturing process.
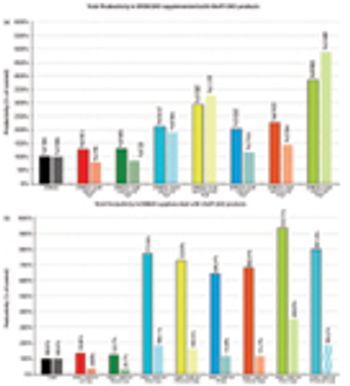
Cell-line specific complex media supplements combine chemically defined media additives into a single supplement.
The authors present scale-up from a 5-L fermentor to a 50-L pilot-scale using the criterion of constant power consumption per unit liquid volume.
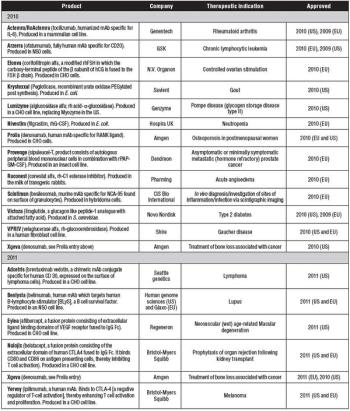
A review of new biologic drug approvals over the years, featuring highlights from 2010 and 2011.

Mike Clayman, CEO of Flexion Therapeutics, talks about his company's strategy to focus on a single therapeutic area.

A review of key industry shifts and promises for the future.

Charles H. Squires of Pfenex discusses advances in expression platform solutions.

Formulators and developers are at the heart of the industry's basic premise-they are saving lives.

Industry experts discuss significant achievements. Plus: What's in store for the future.
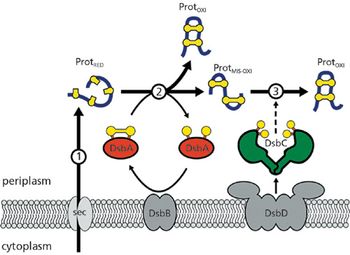
The author highlights novel strains and methods that have recently been shown to express multidisulfide bonded proteins.

This article discusses potential opportunities to improve the patient experience through formulation and delivery device technologies.
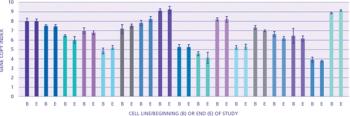
The author describes expression technology that produces cell lines with high genetic stability.
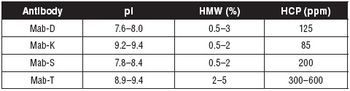
MAb polishing using salt tolerant interaction membrane chromatography.

A Q&A with Alan Shaw of Vaxinnate. This article is part of a special section on expression systems.
Industry experts discuss the benefits and challenges of self-administration of injectable therapies.

A technical rountable featuring Sartorius Stedim Biotech, Pall Life Sciences, 3M Purification, Asahi Kasei Bioprocess, and Bio-Rad Laboratories.

The authors describe a new assembly for bulk and final drug product filling operations.

BioPharm talks with Tarja Mottram, CEO of Action for Results, about key business considerations when starting out.
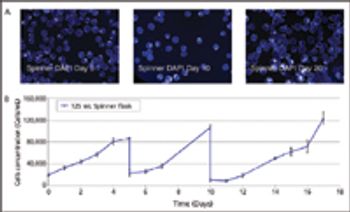
Demonstration of large-scale stem-cell scale-up.
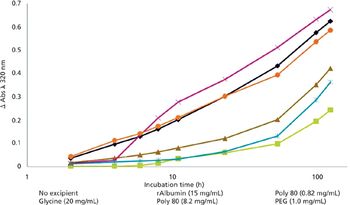
Recombinant albumin can stabilize a drug product and assist in API release.