
A water distribution plan is at the heart of an overall energy and water conservation program.

A water distribution plan is at the heart of an overall energy and water conservation program.

Like any other financial venture, investing in PMPs requires both the patience of a saint and the ability to absorb some bad news and still hang in there.
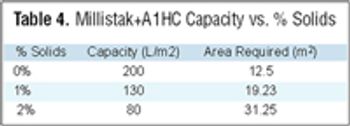
Filtration is one of the most commonly used unit operations in biopharmaceutical manufacturing. Available formats include direct or normal flow filtration (NFF) and cross or tangential flow filtration (TFF). These methods are used for sterilization and virus filtration, depth filtration or ultrafiltration, and diafiltration applications. Some common objectives include:
Synthetic ligand adsorbents can eliminate the potential hazards of animal- and microorganism-sourced ligands, minimizing running, cleaning, and validation costs.

There is no current capacity crunch due to increased investments in new facilities.

Although sounding like a singular term, a water purification system actually consists of several key components.

Green plants and production systems that are most removed from conventional commercial food and feed crops are most likely to succeed
This technology includes an efficient and scalable in vitro enzymatic amidation step for peptide hormones.

The debate over drug importation is a prime example of politics trumping science as members of Congress reject the consensus of scientists and policy experts that opening US borders to therapies from abroad raises serious public health concerns.

Although there are many differences between the industries, especially related to regulatory requirements, there are enough similarities that the future of biopharmaceuticals with respect to contract manufacturing might look much like the semiconductor industry.
Is it safe? Answering that question for therapies based on living cells is not simple.

The employer's first step in considering whether to use non-competition and non-disclosure agreements is to assess, analyze, and understand the benefits of such agreements.

According to a 2002 report by the consulting firm BioPerspectives, the protein biochip market will reach $700 million in sales by 2006.
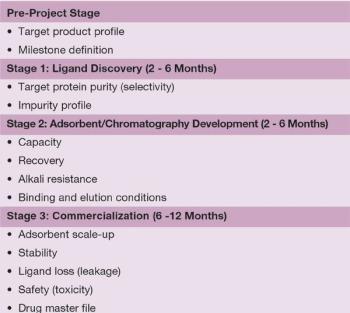
Very significant progress has been made since the mid-1970s when dye ligands were first introduced.

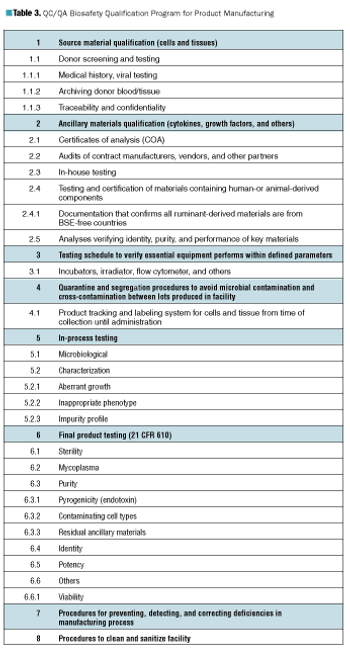
The approaches taken to containing the risk of BSE reveal patterns in the difficulties of performing and reacting appropriately to risk analysis.

Commercial embodiments of genetically modified inventions are protected in Canada.

The Madrid Protocol offers companies another option for trademark registration on a global scale.

Carefully prosecuted patent applications lead to patents with valid and enforceable claims that provide broad coverage for your technology.

Biotechnology players are emerging in countries that historically have not been substantially active in this industry.

FDA's regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures provides industry with the requirements that allow electronic records and signatures in computerized systems in place of paper records and handwritten signatures.1

There are six mechanisms for bringing a veterinary biologic into use when circumstances demand a rapid response and full licensure will take too long.

Introduction - Gene therapy is a medical intervention based on the modification of the genetic material of living cells. Currently, gene therapy is restricted in application to somatic cells.

Spreadsheet calculations are popular in all kinds of businesses. A macro is a set of commands that can be embedded in a document to add functionality to standard programs and to automated processes.

It may take take more than a decade to bring a new drug to market, but it's still a race from the bench to the shelf. It's easy to think of the race in terms of a sprint, like a six-second dash from one end of the pool to the other, especially during crunch times when you're rushing to meet deadlines. However, developing a biologic drug is a much longer race, more like a 400-meter medley. If you've ever competed in one, you know it's a test of endurance that requires a long-distance mind-set, strategic thinking, and a complex combination of skills.

The European Union requires final container testing of US-manufactured biopharmaceutical products to be performed in Europe for release into the European market. Similarly, but less strictly enforced, the US requires final container testing in the US for European-manufactured biopharmaceutical products before release.