
IT, payroll, manufacturing, and clinical-trial data management are key areas for growth in biopharm outsourcing efforts.

By the Numbers: What it Costs to Operate a Biopharmaceutical Facility

IT, payroll, manufacturing, and clinical-trial data management are key areas for growth in biopharm outsourcing efforts.

Health programs seek to establish secure systems to manage the fast-expanding quantities of drugs and medical products going to developing countries.

The Senate confirmed Lester Crawford in July as the official head of the Food and Drug Administration (FDA). Crawford's nomination had been put on hold as various legislators pressured FDA to approve an over-the-counter version of the "morning-after" pill, Plan B, and to support broader drug importing.

Formal process and operation improvement activities are being employed in almost every biopharmaceutical manufacturing company, according to a recent survey conducted by Tefen Ltd and Millipore Corporation. The industry-wide survey was conducted to assess current biopharmaceutical operations excellence (OpEx) trends and needs, as well as OpEx perceptions and expectations related to industry suppliers.

...biopharmaceutical companies still face rampant piracy and counterfeiting of patented products.

Daunting but common challenges currently face many biotech, pharmaceutical, and device firms. These companies are encountering a restless public, worried investors, and a skeptical, publicity-hungry Congress that are all concerned about product safety and the reliability of regulators' scrutiny.

Current best practices of containment reduce the risks associated with biotech development.

Pharma industry equipment utilization hovers below 40 percent, which would be an unacceptable figure in most industries.

Part of the problem with cancer drugs as investment picks is that investors don't trust the underlying studies that seem to prop up vaccine maker stocks.

Historically, the big pharmaceutical companies (Big Pharma) have sought to feed their marketing machines by manufacturing blockbuster drugs—chemical-based, one-type-fits-all products that treat chronic conditions such as heart disease or arthritis. This approach has yielded recurring revenue streams from large patient populations. In contrast, biotechnology companies typically have created protein-based drugs,or biologics, to treat acute or niche conditions and diseases. With few exceptions (such as the biotech giant Amgen), biotech companies have foregone doing the marketing and sales of their drugs themselves, and have, instead, relied on others to perform their marketing and sales functions.

The Interface of America's Antitrust and Intellectual Property Laws

Patents are litigated in the biopharmaceutical industry perhaps more often than any other form of IP

Trademark protection in the US is based on a dual system of federal and state laws.

Biopharmaceutical companies need to consider intellectual property issues early on, even at the start-up stage.

Your research and development team has just shouted "Eureka!" after long and expensive years of research, exclaiming they have developed a next-generation pain reliever. What do you do next? This article explores and suggests your next steps and identifies pertinent questions to ask a patent attorney. The focus is on intellectual property; this article does not address the myriad regulatory issues that must be resolved.

Presenting documentation in a timely manner will expedite the inspection process and provide a positive impression to agency representatives.

It's summer and the living is easy if you don't mind heat, insects, and thunderstorms. The biopharm indexes are listless, because August and September are notorious for inactivity in the various life sciences stock market indexes. Earnings announcements taper off and life sciences companies keep their gunpowder dry by holding off new announcements until after Labor Day. As of mid-July, the American Biotech Index (Symbol: BTK) has leveled off at the tail end of a year of unbridled growth. If history is any indication, the autumn months will see another rise from the 550 or so levels we're now seeing in the BTK.
The pharmaceutical and biotechnology industries are at a critical point in their evolution. Industry experts at Datamonitor, an independent market analyst firm, say that in 2002 about $30 billion worth of blockbuster drugs lost patent protection. By 2008, an additional $35.5 billion worth of products is expected come off patent. At the same time, a host of scientific innovations in drug discovery including the use of high-throughput screening techniques, new classes of therapeutics such as aptamer technology and RNAi, and genomics-driven discovery methods, have resulted in large numbers of new drug candidates.

Subcutaneous administration is likely to be an important factor in generating an immunogenic response.

A more recent challenge for CBER is to ensure the safety of an expanding number of tissue and cellular products used in transplants.
Misinterpreting the effluent profiles obtained during tracer measurements performed for determining packing quality can often lead to excessively large percolation velocities and exaggeration of packing problems. Highly useful and reliable information can be obtained through characterization of tracer effluent curves using the method of moments, information that could be critical for successful scale-up of chromatographic steps. This is the sixth in the "Elements of Biopharmaceutical Production" series.

I was playing tennis recently with my friend Ron who, in addition to a wicked backhand, is well-versed in new technologies and gadgets.

FDA plans to conduct good manufacturing practices (GMP) inspections of flu vaccine manufacturers annually instead of every two years.

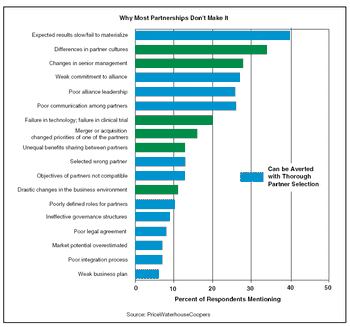
One of the very last sessions on the very last day of BIO 2005 drew quite a crowd. It was titled, "The Five Worst Mistakes You Can Make When Creating a Collaboration or Strategic Alliance."

When data are not normal, a more efficient approach to monitor and control the performance of this assay requires transforming the data to a normal distribution. One of the most useful transformations was invented by Taguchi.