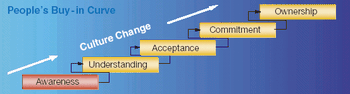
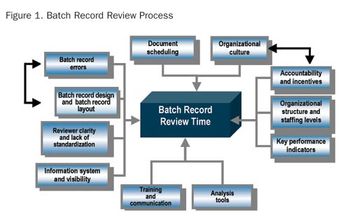
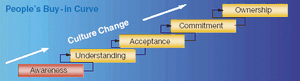
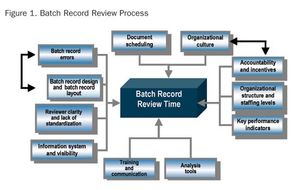
Formal process and operation improvement activities are being employed in almost every biopharmaceutical manufacturing company, according to a recent survey conducted by Tefen Ltd and Millipore Corporation. The industry-wide survey was conducted to assess current biopharmaceutical operations excellence (OpEx) trends and needs, as well as OpEx perceptions and expectations related to industry suppliers.