
The least we can do in our industry to ensure scientific integrity in our pursuit towards effective CAPA is to have our data peer reviewed.

The least we can do in our industry to ensure scientific integrity in our pursuit towards effective CAPA is to have our data peer reviewed.

In March of 1932, socialite and millionaire playboy Eben M. Byers died of a horrible and mysterious disease, his body corroded until his bones began to shatter apart. Strikingly handsome in his youth, by the time of his death a series of last ditch operations had eliminated the lower part of his face and reduced him to a distorted shadow of his former self. His bizarre death was the result of his addiction to a quack cure, radium-laced water.

As the bustle of Sarbanes-Oxley (SOX) 2004 compliance deadlines for companies winds down, executives have an opportunity to reconsider their company's compliance strategy.

Last year's catastrophic flu vaccine shortage and escalating Congressional debate over drug safety continue to shine the spotlight on biotech product manufacturing. FDA officials are under pressure to address concerns about access to quality biotech products, while also encouraging the development of new treatments to meet patient needs.
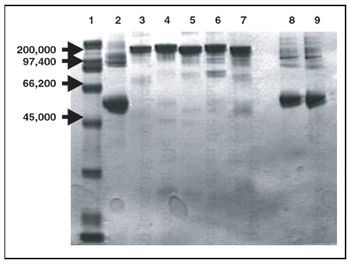
An anionic column with modified chitosan bead matrix performs well in purifying cell culture. A pair of cationic-exchange columns shows promise in purifying S25 antibody.
Utilizing a measured approach can increase the probability for identifying search criteria that fulfill a company’s strategy.
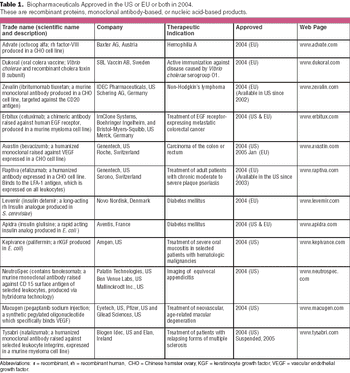
Cancer remains the primary indication (three products), again mirroring recent approval trends.
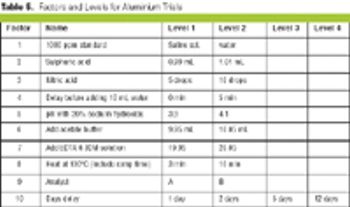
Saturated fractional factorial plans minimize the number of trials by one-half or better, which saves time and money.

Despite the ringing of two stray cell phones disrupting the quiet, all eyes and ears were glued, almost reverently, on New York City's former Mayor Rudy Guiliani at INTERPHEX when he presented the day's keynote address. First, he thanked the audience for providing the therapeutic options that allowed him to successfully defeat prostate cancer awhile back. Next came his imitation of James Gandolfini in the hit TV show "The Sopranos," as he reminisced about his duties as NYC's former chief prosecutor. Once he had our full attention, close to 1,000 of us, he shared what he felt are the most valuable leadership attributes and how they will successfully get you through challenging and uncertain times.

Three meetings of the BioPharma Operations Excellence Consortium, facilitated by Tefen Operations Management Consulting, were held recently. The east coast meeting was hosted by Genzyme at its headquarters in Cambridge, MA; the west coast Chapter met at the Genentech campus in South San Francisco, CA; and the European forum held its meeting in Frankfurt, hosted by Aventis.

$200 million and two years could be shaved off a drug's development time by using informatics effectively.

Geron rose 7% and StemCells 15% . . . when news of the New York capital funding effort hit the business pages.

FDA will allow nonprescription NSAIDs to share the market with products carrying black-box warnings.

You don't really care what people say about you (the biopharm industry) behind your back, do you? According to a recent survey of 670 BioPharm International subscribers, conducted by Derek Ellison from Eden BioDesign, some of you care very much about the public's perception of the industry.
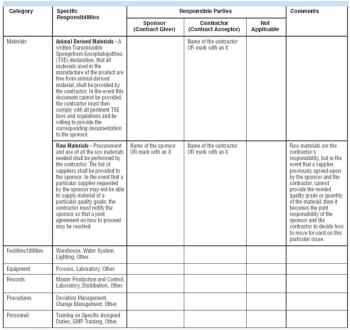
During the past several years in the pharmaceutical and biopharmaceutical industries, conflicts and misunderstandings have arisen between companies and their contractors. Too often, productive working relationships have crumbled, resulting in expensive production delays with companies and contractors squabbling over their roles and responsibilities. Such conflicts may have their roots in the lack of a sound quality agreement (QAG). QAGs that clearly delineate good manufacturing practice (GMP) responsibilities between a sponsor and a contractor can help companies and their contractors avoid certain conflicts.

Mass serialization, or the ability to store a unique serial number for each item, is the most useful feature of RFID tags.

Even if some clinical testing is needed, generics makers argue that no one-size-fits-all testing approach is appropriate for the broad range of biopharmaceutical complexities.

Biotechnology and life sciences companies come in all shapes and sizes. Some are multinational companies with vast resources and others are small companies working with a few new compounds. Regardless of size or market position, these companies should all have one common question of those that handle their insurance: Will their current insurance program protect their assets and investments in the event of a significant loss? Understanding the nature of risks, acquiring suitable insurance, and comprehending policy issues when claims arise are essential to protecting assets and obtaining reimbursement when losses occur.

With biotech valuations at the lowest point in years, the resulting investment opportunities are multiplying - seemingly by the hour.

Creation and qualification of scale-down models is essential for performing several critical activities that support process validation and commercial manufacturing. This combined article is the fifth in the "Elements of Biopharmaceutical Production" series. Part 1 (March 2005) covered fermentation. In this segment, we present some guidelines and examples for scale-down of common downstream unit operations used in biotech processes - chromatography and filtration.

When you don't know the answer to a question, ask an expert. If the question is really big, ask more experts. If you have a collection of difficult questions, run a poll of many experts. That, in effect, was the impetus for Eden Biodesign to survey 670 BioPharm International subscribers with questions as to what will be the development mechanism to achieve safe, effective, and cheap new medicines.

Out-licensing has become a crucial part of most biotech companies' business strategies.

This was the week for awards. The Academy Awards, which honored the film industry, captured the eye of millions worldwide. They were followed midweek by The Fourth Annual Top 25 Direct-to-Consumer (DTC) Marketers of the Year Awards. Sponsored by USA TODAY and DTC Perspectives magazine, these awards celebrated the accomplishments of 2004's most talented DTC pharmaceutical marketers.

Analytical method validation (AMV) is required in the biopharmaceutical industry for all methods used to test final containers (release and stability testing), raw materials, in-process materials, and excipients. 1 AMV is also required for test methods that are used to validate the process prior to process validation. This article reviews current regulatory guidelines and the critical elements of analytical method development (AMD) that should be finalized before starting AMV.

Synthetic drugs can be well characterized by established analytical methods. Biologics on the other hand are complex, high-molecular-weight products, and analytical methods have limited abilities to completely characterize them and their impurity profiles. Regulation of biologics includes not only final product characterization but also characterization and controls on raw materials and the manufacturing process.