
There are challenges aplenty in purification and analysis of PEGylated protein pharmaceuticals. Here are a variety of technical solutions, many concentrating on the chemistry of the linker.
The BioPharma Operations Excellence Consortium, facilitated by Tefen Operations Management Consulting, continues to thrive, recently holding chapter meetings on two separate continents. The US East Coast Chapter met at Centocor's headquarters in Malvern, PA, while the European Chapter met at Sorono's facility in Vevey, Switzerland. Since its establishment in early 2002, over 45 leading biopharmaceutical companies have joined the forum, which operates on the basis of using the group's collective knowledge to drive each member company - and the industry as a whole - to world-class levels of operational effectiveness and efficiency.

There are challenges aplenty in purification and analysis of PEGylated protein pharmaceuticals. Here are a variety of technical solutions, many concentrating on the chemistry of the linker.

The variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method for validating all types of tests. By their very nature, microbiological tests possess properties that make them different from chemical tests. Consequently, the well-known procedures for validating chemical tests are not appropriate for many microbiological tests. Yet, it is necessary to validate microbiological tests if they are to be useful for controlling the quality of drug products and devices. Test-method validation provides assurance that a method is suitable for its intended use. Given this definition, any rational company would want to be sure that its methods are validated.

Revisiting simple, robust, and controllable technology is the only way to overcome these challenges.

Lot-to-lot variations between raw materials can greatly impact process performance.

A tepid currency picture may cause biopharm companies to put the breaks on initiatives in places like information systems, research and development, and even hiring.

The purpose of design validation is to demonstrate that a product performs as intended. The usual route to this goal is showing that every item on the specification has been achieved, but it is not an easy path. The specification itself can create difficulty if it includes statements like "as long as possible" or the real horror "to be decided." Verification tests can reveal so many problems that the design must change to such an extent that earlier tests are no longer relevant. And there is also the practical difficulty of obtaining sufficient samples to test when the manufacturing engineers have not completed their standard operating procedures, the product design is not fixed yet, the component suppliers are late, and the marketing department has taken all the samples to show to prospective customers.
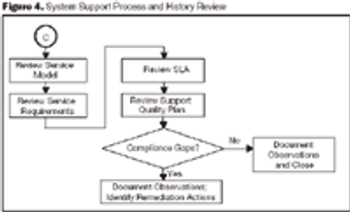
Organizations can mitigate the risk of failure during regulatory inspections by identifying and correcting deficiencies before an inspection occurs.

In most production environments, it is critical to be able to associate historical environmental data with process data.

One of the critical early tasks of the documentation team is coordinating plans and requirements with the start-up team.

The growing alarm over harmful side effects from a number of popular prescription drugs is affecting a range of issues of critical importance for pharmaceutical and biotech manufacturers. Safety concerns may slow down efforts to expand drug importation from foreign nations. The National Institutes of Health (NIH) has halted important clinical trials due to fear that the painkillers under study increase risk for cardiovascular events.
I wrote in the December 2005 issue of Streetalk that no matter who won the election between George Bush and John Kerry, the result would have been good news to the biopharm industry - if for different reasons. I said that with President Bush back in office for a second term, industry red tape would be diminished and the life sciences sector would shift into higher gear.

In the first part of this feature (Jan. 2005) we discussed the technical background and the role that FDA, US Department of Agriculture (USDA), and Environmental Protection Agency (EPA) play in setting the rules for accepting plant-made biopharmaceuticals (PMBs). We now continue by discussing how producers will be able to take products to market.

Unfortunately, once circuits are commissioned and validated, optimizing - or even adjusting - them is difficult and rarely done because of the time and effort required for revalidation.
The bulk of a biopharmaceutical processing unit can be assembled with off-the-shelf components. However, special fabrications — especially fluid components — enable fabricators and manufacturers to meet critical construction deadlines and move projects forward with minimal or no delays.

The drive to develop better, faster, and smaller - in other words, more efficient - products is a universal trend in the modern world. This trend has profoundly impacted many industries from microelectronics to packaging equipment. In the biopharmaceutical industry, the need to speed and simplify the long and complex drug manufacturing processes brings additional challenges, such as meeting regulatory requirements.

Manufacturing changes - such as changes in formulation or source material - can impact a product’s immunogenicity.

In order to institute a GxP mindset across the organization, support and respect for quality systems should come from the top down.
You don't need me to tell you how bad the news has been for the pharmaceutical industry in the last two months. First, it was antidepressants for children, then Vioxx. It took maybe 48 hours before the first tort request hit the airwaves, asking to hear from those who'd experienced heart attacks or strokes after taking the drug. Celebrex came next, followed by NIH announcing it put the kibosh on a study of naproxen.

In the increasingly competitive world of drug discovery and development, the role of branding is more important than ever. With all the various sectors - big pharma, generics, specialty pharma, and biotechnology -vying for limited dollars, branding must begin early in the development process and, ultimately, play an integral role not only in taking a product to market, but also in sustaining that product against competition, perceived and real.

Transgenic plant systems promise lower production costs since plants can be grown in a field instead of a cell-culture facility. Also, they provide an alternative production system for proteins that are difficult to express in cell culture.

The re-election of George W. Bush as president and continued Republican control of Congress will shape the healthcare policy agenda for the coming year. Major initiatives to expand healthcare coverage to the uninsured or reform Medicaid are off the administration's priority list, but social security and tax reform may provide additional tax incentives for individuals and small businesses to obtain health coverage and establish health savings accounts.

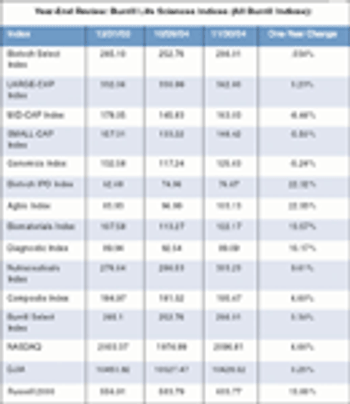
For years, the pharmaceutical sector has provided a safe haven for investors. I'm not so sure that will be the case in 2005.

Biopharmaceutical companies are developing clever technologies for plant made pharmaceuticals (PMPs). When will the promise for the future turn into reality?

The last few months of 2004 were marked by crises likely to affect pharmaceutical and biotech manufacturing for years to come. Safety data indicated that antidepressants may cause problems for young patients that warrant black box warnings, new labeling, and special packaging. Merck pulled its blockbuster COX-2 inhibitor Vioxx off the market because studies showed increased risk of cardiac events from long-term use of the drug. To cap it off, British regulators shut down Chiron's Liverpool vaccine plant due to product contamination, creating a serious shortage of flu vaccine in the US.

Immune responses to foreign proteins are expected and may be anticipated for some self-proteins. The rapidity of development and the strength and persistence of the response depends on many factors.