
QbD for QA? Try a two-step approach

Stephan O. Krause, PhD, is the director of quality at Mpex Pharmaceuticals, Inc.

QbD for QA? Try a two-step approach

The industry needs to open up to validation failures.
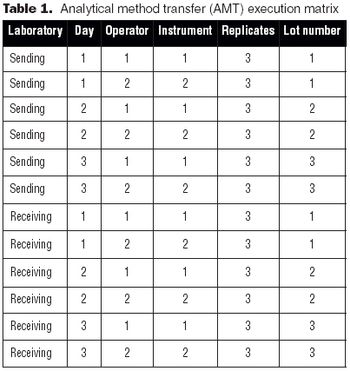
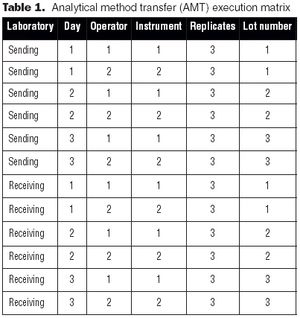
It is essential to understand the critical elements of validation extensions to ensure accurate process or product quality measurements.

The first part of this article, published in the September 2006 issue, discussed general strategies for validation extensions to other test method components, laboratories and even different test methods.1This second part provides practical tips on how to maintain test method suitability long after the formal completion of analytical method validation (AMV) studies.

Good risk management tools dictate how much assay performance characteristics can deviate from ideal.

Many industry professionals know that analytical testing for biopharmaceuticals for all raw materials, production in-process stages, and final containers must be validated, and they generally understand how this can be achieved. Many of us even understand the basic concepts of laboratory compliance and production process quality. However, how exactly are analytical test method performance and process robustness related and how do they depend on each other? Furthermore, how do we monitor and maintain the accuracy and reliability of analytical methods long after validation completion to ensure the suitability of these methods for measuring process quality?

FDA and regulatory agencies worldwide have recently approved many advanced bioanalytical technologies. Receiving approval of advanced test methods for new biopharmaceutical products is relatively straightforward, provided clinical and process validation data are generated by the same (or at least similar) test method. However, regulatory approval becomes more difficult and time consuming when compendial test methods or test methods for already licensed biopharmaceuticals are changed.
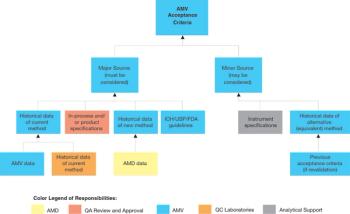
When replacing an existing method, it may be necessary to compensate for a significant difference between the current and new method by adjusting the specifications.

The relationship between "valid" or "suitable and validated" is often overlooked, but there is a high price when "validated" test systems are simply inappropriate.

The European Union requires final container testing of US-manufactured biopharmaceutical products to be performed in Europe for release into the European market. Similarly, but less strictly enforced, the US requires final container testing in the US for European-manufactured biopharmaceutical products before release.
Published: February 2nd 2008 | Updated:

Published: February 1st 2009 | Updated:

Published: June 1st 2008 | Updated:

Published: October 1st 2006 | Updated:

Published: October 1st 2005 | Updated:

Published: September 1st 2006 | Updated: