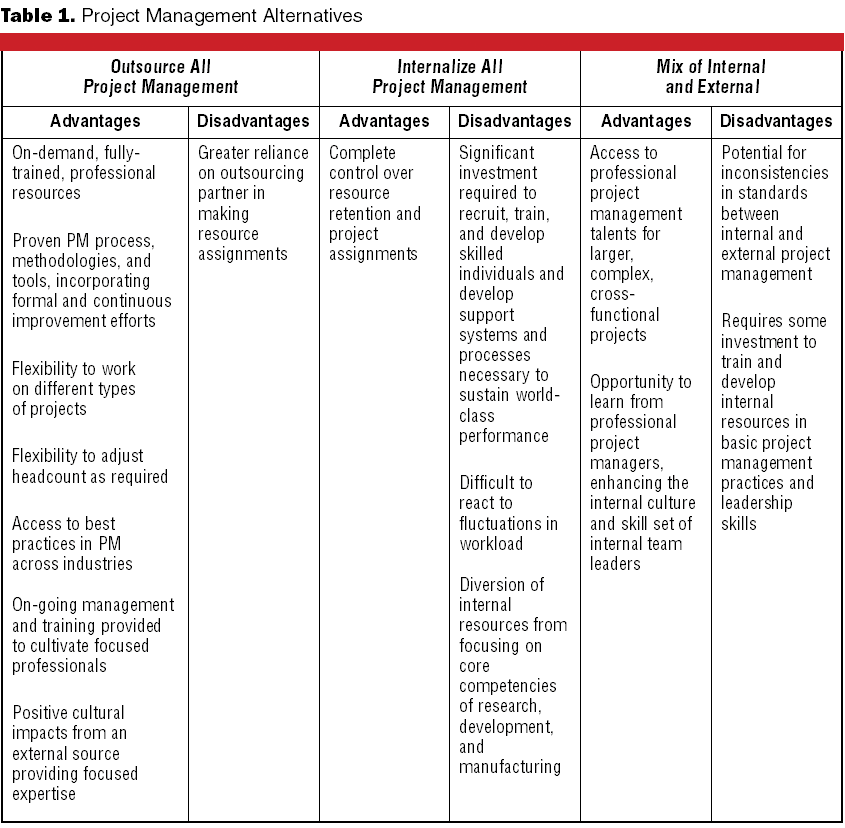
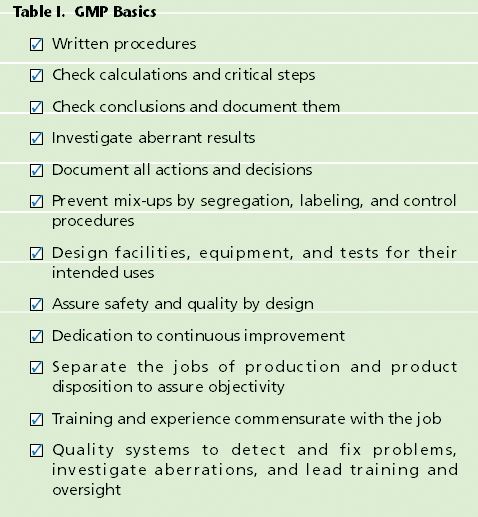
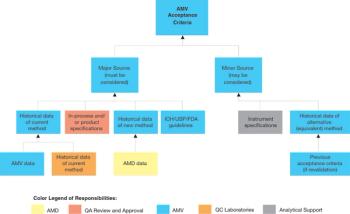
There continues to be much interest within industry and FDA about the future of the GMPs. Discussion groups have been spawned within professional organizations and at FDA to reevaluate the aging GMPs and associated guidance documents to ensure that the government does not impede technological progress, focuses its resources effectively, and upholds its mandate to protect the public.