
Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

FDA announced the recall, citing deficiencies in Medistat’s aseptic processing areas and in its environmental monitoring procedures.

The European Medical Contract Manufacturing (EMCM) organization announced it will team up with VCC Medical NV for a unique personalized medicine initiative-the aseptic manufacture of a biologic therapeutic from the tissue of a patient’s tumor.
Focusing on niche and specialty service offerings gives contract biomanufacturing organizations an opportunity to differentiate in a crowded market.

When should CMOs and their pharma clients share the details of their partnerships with outside parties?
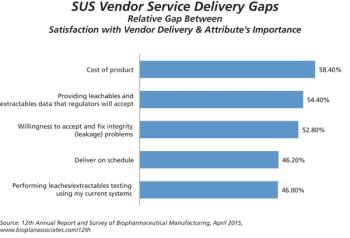
Suppliers indicate prices for single-use equipment are likely to increase.

WuXi's Laboratory Testing Division will be the exclusive supplier of laboratory testing services for Hong Kong-based Lee's Pharm.

Cytovance Biologics anticipates continued expansion plans following acquisition by Hepalink USA

*This article is an opinion piece and does not necessarily represent the views of BioPharm International.

The agency issues guidance for companies considering registering with FDA as an outsourcing facility.

Biopharma companies are outsourcing more jobs to cut costs.

Malvern Instruments' biophysical characterization equipment will be installed in a commercial applications laboratory in San Diego, California.

CMC Biologics and River Vision Development announce manufacturing agreement for RV001, a monoclonal antibody to treat Grave’s orbitopathy.

Cytovance Biologics Inc. announces development and manufacturing deal with NeuroFx for stem-cell-derived cell-free therapy for strokes.

Financial expectations for Baxter’s biopharma solutions arm will drop 10% as the result of a client’s decision to move its manufacturing in house.

The $19.7 million contract will assist Emergent with the development of cGMP lots of three Ebola mAbs.

Baxter has initiated a voluntary recall of two lots of IV solutions due to the potential presence of particulate matter.

Evans Analytical Group expands into the pharmaceutical/biopharmaceutical industry with acquisition of ABC Laboratories.

The new project will increase fill/finish manufacturing capacity for select products on FDA’s Drug Shortage Index.

Catalent’s licensing of Excelimmune’s antibody combination therapy platform can enable more consistent, cost effective production of antibody combinations.

The addition of a new manufacturing line at Lonza’s Portsmouth, NH site enables Alexion to add dedicated product supply for 10 years.

The new facility expands the company’s commercial manufacturing capability at its Bend, Ore. site.
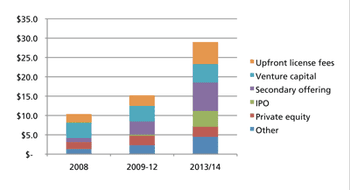
While all market signs are pointing up, memories of past setbacks may discourage CDMOs from expanding capacity.

Gil Roth, Founder and President of the Pharma & Biopharma Outsourcing Association speaks with BioPharm International.

Jim Miller, President of PharmSource, speaks with BioPharm International.