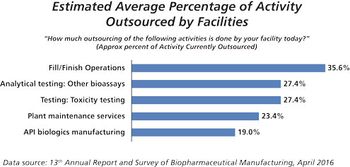
This key bioprocessing segment is expecting continued growth.

This key bioprocessing segment is expecting continued growth.

The strategies of a innovation-driven CMO may be different than a capacity-driven CMO.

Agilent Technologies announces plans to build a new oligo manufacturing facility in Colorado that will double current capacity.
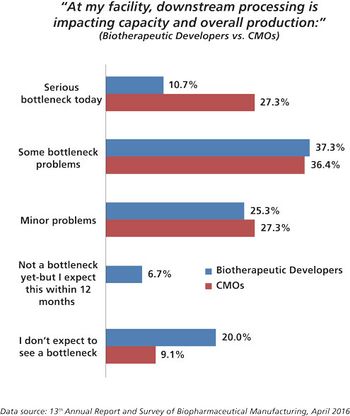
CMOs are working hard to improve performance by investigating new technologies for filtration and purification.

GenPak Solutions is cited by FDA for cGMP violations at its Hilliard, Ohio facility.

EAG Laboratories announces new company identity and intent to expand testing, analysis, and characterization capabilities across multiple markets.

Patheon launches initial public offering to repay outstanding notes and expenses.
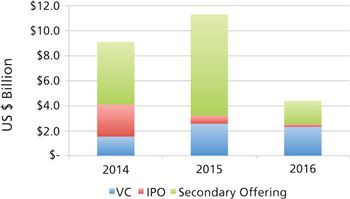
CDMOs need to be aware that unfavorable public markets put emerging bio/pharma R&D spending at risk in 2017.

Capacity for complex therapeutics is become increasingly difficult to predict.

Technology advances enable contract service providers to keep pace with the demands of existing and emerging biologic-based therapies.

Contract service providers debate the pros and cons of consolidation, accelerated drug approvals, regulatory issues, and the impact of new therapies.

Planning ahead is key to enabling a continuous and secure supply chain that adapts to changes in market demand.

For successful drug delivery partnerships, it’s important to take a long-term view, focus on simple designs, and address potential payer concerns up front.

New drug approval policies promote industry growth and injectable contract manufacturing opportunities in China.

Biopharmaceutical manufacturers continue to put quality at the forefront of their relationships.

Adopting best practices and collaborating more closely with contract research organizations can help drugs get past Phase III.

Biopharmaceutical contractors continue to invest in development, facility upgrades, technological advancement, and mergers and acquisitions.

Brammer Bio establishes late-phase development and commercial manufacturing facility for advanced cell and gene therapies in Lexington, MA.

The new company is the product of a merger with Formex.

Uncertainty about the demand for a biologic medication can be partly mollified with some well-planned capacity outsourcing, contends a new report by ORC International sponsored by Patheon.

Acquisition binges often lead to hangovers; here’s what to watch out for.

Advances in single-use systems, consumables, and continuous manufacturing show steady progress.

BioPharm sat down with an intellectual property lawyer to examine how companies are protected when they engage in activities where sharing of trade secrets must occur.

The company’s method reduces the time required to crystallize antibodies from weeks to one day.

Investment at SGS’s Mississauga, Canada facility provides for analysis of molecular interactions in real time.