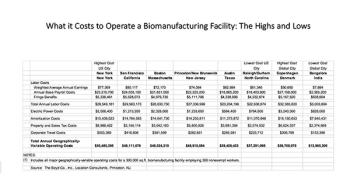
As politicians focus on drug cost reduction, biopharmaceutical companies in the US are moving to states with lower taxes, and relocating some facilities that had been offshore.
Agnes Shanley is senior editor of BioPharm International.

As politicians focus on drug cost reduction, biopharmaceutical companies in the US are moving to states with lower taxes, and relocating some facilities that had been offshore.

Vein-to-vein programs are focusing on data access and traceability.
While the US public and law makers push for price controls, pharma’s venture capitalists have other ideas for balancing innovation and affordability.

Focusing on symptoms instead of root causes locks teams into a corrective, rather than preventive, mindset.

Bob Lenich, Emerson's business director for global life sciences, shares insights on where biopharma modeling is now, and where it is heading in the near future.

Legal experts in biopharmaceutical patent law shed some light on trends and recent news.

Modeling is being used for everything from yield improvement to facility design, but new initiatives plan to broaden its reach, both upstream and downstream.

Ireland's National Institute of Bioprocessing Research and Training (NIBRT), together with technology providers such as GE Healthcare, and universities in Ireland and around the world, are developing courses that aim to close the gap between theoretical studies and practical workplace needs.

New guidance from FDA, including standards for interchangeability, is expected to speed development of biosimilars, but experts warn against oversimplifying risk to reduce costs.

Used with perfusion, alternating tangential flow and tangential flow filtration are redefining upstream efficiency.

The agency seeks comments on a draft guidance designed to make the process conform to its original intent as a communication tool, rather than a roadblock to competition and innovation.

Industry practice has changed radically over the past five decades. Can laws published in the 1960s still ensure pharmaceutical quality and safety today?

Personalized medicine and direct-to-patient trial models have made the difficult even more challenging.

Now that the first genetically modified cell therapies are being manufactured, the industry must move beyond “whatever works” to meet growing demand.

Although downstream efficiency still lags behind upstream, engineering-driven innovation is breaking through boundaries.

Making siloed data accessible across functions and to contract partners is the first step to facilitating continuous improvement and enabling use of artificial intelligence in manufacturing.

More manufacturers are embracing MAM, which simplifies biopharmaceutical product quality testing, and facilitates the measurement and monitoring of critical quality attributes.

While it may not be getting easier, biopharma patent protection is at least becoming more predictable.

Technology vendors are strengthening their positions in China as the nation emphasizes self-sufficiency in research, development, and manufacturing.

Radha Iyer, vice-president and head of Quality and Scientific Affairs, Global Developed Markets, Dr. Reddy’s Laboratories, discusses new initiatives at the company.

When transferring a method from R&D to QC, success hinges on discovering where “the best” and “the most reliable” intersect.

Success depends on supplier communication and transparency, but it’s up to buyers to demand the right information and to look at the vendor’s overall business.

While recognizing the unsung work of scientists in corporate research and development, the Galien Awards remind the industry of its priorities: patients and future patients within the global community.
Platform processes have improved monoclonal antibody scale-up. Can they do the same for personalized therapies?

Safeguarding the know-how behind biopharmaceutical innovation is crucial to the industry’s future, but, in the US, some argue it is becoming increasingly difficult to do.

Logistics manufacturers are adapting to explosive growth in the use of direct-to-patient clinical trials. Michael Sweeney, director of patient-centric logistics at World Courier Group, discusses the new model’s impact.

The need to improve and understand processes is moving PAT and more advanced control strategies beyond the lab into manufacturing and downstream applications.

Lower costs, fewer opportunities for temperature excursions, and a smaller carbon footprint are making ocean transport more attractive for pharmaceuticals. Poseidon, a new collaborative pharma initiative, seeks to leverage benefits.

Alan Kennedy, executive director of TEAM UP, shared perspectives on Poseidon and ocean transport.
As closure integrity testing moves from a probabilistic to a deterministic basis, designs are promoting improved control and reduced operator contact.

Published: November 24th 2014 | Updated:

Published: December 8th 2014 | Updated:

Published: June 15th 2015 | Updated:

Published: March 1st 2016 | Updated:

Published: March 1st 2016 | Updated:

Published: April 1st 2016 | Updated: