Nothing beats a good dictionary. It can clarify doubts, settle an argument, or prompt exploration into new areas of learning.

Nothing beats a good dictionary. It can clarify doubts, settle an argument, or prompt exploration into new areas of learning.

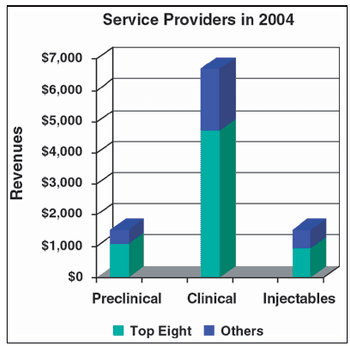
Many CMO relationships are established while a product is early in clinical or preclinical development.

Disposables require less space than conventional equipment, and they can be assembled offsite into complete process trains.

The lack of biomanufacturer acquisitions seems surprising.

The top 10 Chinese companies in the biopharm sector account for only 20% of the market... leaving plenty of room for growth for Western companies.

The foundation of a sound supply chain planning infrastructure requires implementing a strong, systematic forecasting process.

Interestingly, it is companies that already have the most capacity available to them that are building even more.

Moving aggressively to implement its Vision 2010 strategy, which debuted in October 2005, DSM N.V. (Heerlen, Netherlands) announced in December 2005 that it would shut down its Montreal biomanufacturing facility in early 2006. The move doesn't signal an exit from manufacturing, however, but a change in focus; DSM will simultaneously expand its expression-technology relationship with Crucell (Leiden, the Netherlands). Both moves reflect changing circumstances in the biomanufacturing sector.

Before designing cleaning procedures, it's vital to know all physical and chemical characteristics of the product ingredients.

These latest pressures on technology relate not only to the need for improved manufacturing productivity and shorter development times, but also to the need to create smarter manufacturing operations

Achieving approval of a new pharmacologic agent or device on a worldwide basis is a significant challenge. The guidelines and requirements that steer our efforts at enhancing and extending life around the world are tedious and, fortunately, comprehensive. In spite of relatively few setbacks, perhaps in no other category than pharmaceutical development are the advantages of these guidelines more evident.

Simple practices can increase profit margins and ensure gas supply.

IT, payroll, manufacturing, and clinical-trial data management are key areas for growth in biopharm outsourcing efforts.

In addition to India and China, other Asian countries are establishing themselves as destinations for biopharma companies.
Avariety of forces are combining to fundamentally change the financial dynamics of the biopharmaceutical industry. The initial public offering (IPO) appears to be giving way to licensing arrangements with, and acquisitions by, major pharmaceutical companies.
Less than 35 percent of all biotech companies have sufficient finances to survive beyond one year.
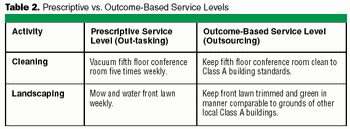
Your company's job is to make biopharmaceutical products. Managing facilities is a function supporting the main task. General manufacturing companies discovered this long ago, but pharmaceutical producers have been lagging. Once you consider the outsouring of non-core activities like facility management (FM), office services, space planning, and utilities management, you can focus on core business functions that make profits.
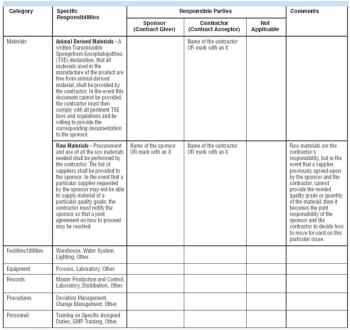
During the past several years in the pharmaceutical and biopharmaceutical industries, conflicts and misunderstandings have arisen between companies and their contractors. Too often, productive working relationships have crumbled, resulting in expensive production delays with companies and contractors squabbling over their roles and responsibilities. Such conflicts may have their roots in the lack of a sound quality agreement (QAG). QAGs that clearly delineate good manufacturing practice (GMP) responsibilities between a sponsor and a contractor can help companies and their contractors avoid certain conflicts.

Mass serialization, or the ability to store a unique serial number for each item, is the most useful feature of RFID tags.
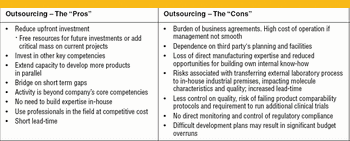
Outsourcing is becoming increasingly widespread and essential in the biopharmaceutical industry. Its imprint on biotech world business and on the development of biopharmaceutical drugs is becoming ever more pronounced. It is estimated that almost one-half of biopharmaceutical companies contract out at least part of the production of their products. On the other hand, those companies that do not outsource production often contract out some of their development activities.
Contract manufacturers must plan for increased analytical resources in development and quality control.

The contract manufacturing sector has not enjoyed the success of preclinical and clinical businesses.

Speed of response, small-scale manufacturing and process flexibility will become increasingly important.
The bulk of a biopharmaceutical processing unit can be assembled with off-the-shelf components. However, special fabrications — especially fluid components — enable fabricators and manufacturers to meet critical construction deadlines and move projects forward with minimal or no delays.

In today's competitive and fast-paced environment, owner companies must get products to market quickly and efficiently. By redefining how they provide services and products, suppliers can enhance their value and help owner companies reap the greatest financial returns.