
Abzena has entered into a Master Services Agreement with a US biotech company to provide process development and manufacturing services to progress a novel antibody-drug conjugate (ADC) to clinical trials.

Abzena has entered into a Master Services Agreement with a US biotech company to provide process development and manufacturing services to progress a novel antibody-drug conjugate (ADC) to clinical trials.

The European Medicines Agency has granted Samsung BioLogics approval to manufacture a monoclonal antibody at the company’s second facility in Songdo, Incheon, South Korea.

The new 30,000-L, $150-million biologics manufacturing facility in Wuxi, China, quintuples the company’s existing manufacturing capability.

An increasing number of warning letters shows that FDA is observing more problems with pharmaceutical contract manufacturing.

The contract research, development, and manufacturing organization has expanded API aseptic manufacturing capacity at its Valladolid, Spain, facility.

Biopharma majors are among the industry stakeholders who have commented and raised questions about FDA’s recently proposed draft guidance for analytical assessment of similarity in biosimilars.

Lonza has entered into strategic license agreements for exclusive rights to a gene-therapy platform for developing treatments for hearing and balance disorders.

In a productive year, 2017 was filled with acquisitions, facility expansions, and new biopharma technology.

Biopharma employees reveal employment objectives, opportunities, and frustrations.

Developing and retaining qualified employees will test biopharma companies and CMOs alike.
The Biomanufacturing Technology Roadmap is accelerating innovative manufacturing strategies for biopharmaceuticals.

Biocad and Sothema Labs have partnered to release cancer-treating biosimilars into the North African market.

The acquisition gives GE Healthcare access to a nanofiber-based platform purification technology that can offer improvements in biopharmaceutical productivity.

Germany tops quality ranking in the inaugural CPhI Global Pharma Index.

A new facility type integrates next-generation mobile cleanroom systems.

ADC Bio experts warn of impending problems in the ADC pipeline with millions wasted in development costs.

Alcami will manufacture clinical supply of the API for a drug candidate targeting various hematologics and solid cancers.

The companies have established a joint laboratory to develop full continuous processing to manufacture high yields of monoclonal antibodies at reduced costs.

The company has officially opened a local brand office in South Korean to support its existing business in the region.

Almac Group will acquire BioClin Laboratories to expand Almac’s analytical service offerings.
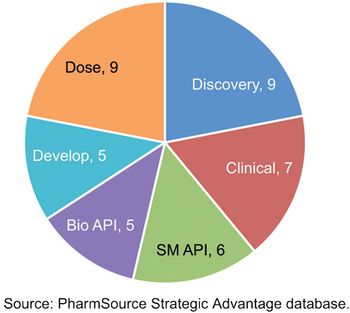
Recent acquisitions are creating CDMOs with scale that rivals global bio/pharma.

The M&A advisory firm has developed an interactive map of manufacturing sites to give insight into the market size of global manufacturing.

ABEC, an equipment and engineering company, will provide a custom-made, single-use bioreactor to custom manufacturing firm, Emergent, for its Maryland manufacturing facility.

The API manufacturer has announced that it has completed the expansion of large-scale manufacturing capabilities at its Charles City, Iowa site.

Samsung BioLogics’ second facility adds 152,000 L of mAb drug substance capacity.