
The article reviews strategies for firms with or without existing in-house capacities and the pros and cons for outsourcing bio/pharmaceutical development and manufacturing.


The article reviews strategies for firms with or without existing in-house capacities and the pros and cons for outsourcing bio/pharmaceutical development and manufacturing.

Effective communication between contract manufacturing organizations and pharmaceutical company clients relies on well-defined master service and quality agreements.

FDA’s focus on the quality culture and its request for quality metrics may ensure a successful company-CMO relationship.

This article reviews experiences with the outcome of in-house audits, audits by third parties, and purchased audit reports.

Operated by BioOutsource, Sartorius’ subsidiary, the Glasgow, UK-based service center will offer physicochemical properties and structural attributes testing and allow clients to perform structural and functional analyses in parallel.

CMOs may be gaining as strategic partners to large bio/pharma companies, but they have a much harder path to navigate.

The funding allows the company to broaden its range of services and finance its move to new laboratory facilities in the Illkirch-Graffenstaden innovation park in northeast France.

Cobra will increase capacity in response to customer demand for DNA and viral vector production.

GE Healthcare continues to ramp up its offerings in the bioprocessing space with the purchase of Asymptote and a continued partnership with Zenith Technologies.
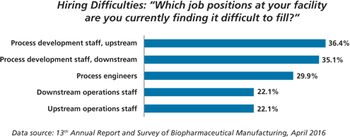
New study shows China biopharma companies face staffing shortages.

Caladrius is selling the remaining percentage of the subsidiary in order to focus on cell therapy development.

The ready-to-fill packaging solutions for vials are based on Ompi EZ-fill packaging design.
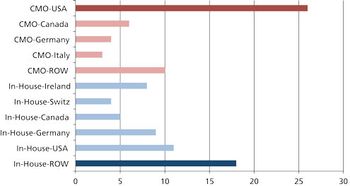
Moving global manufacturing operations may be more complicated than it appears.

Communication and taking the time to develop the process are key to successful transfer and scale up of biologics

Modular Automated Sampling Technology (MAST) allows direct aseptic transfer of bioreactor samples to analytical devices, providing rapid and reliable data in bioprocessing.

The outlook for the CMO and CDMO industry may be affected by ever-changing politics.

SGS invested in test equipment for analyzing extractables and leachables at its New Jersey laboratory.

Vetter expanded visual inspection facilities and controlled-temperature storage at its Ravensburg Vetter West facility in Germany.

The partnership will focus on providing practical information to clients on the development of biologics and vaccines.
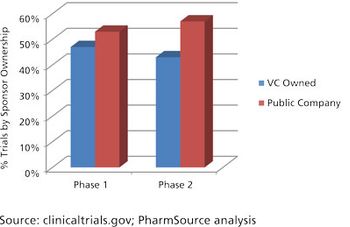
Robust venture capital investment gives CDMOs and CROs a positive outlook for 2017.

AGC adds second biopharma contract manufacturer with acquisition of CMC Biologics.
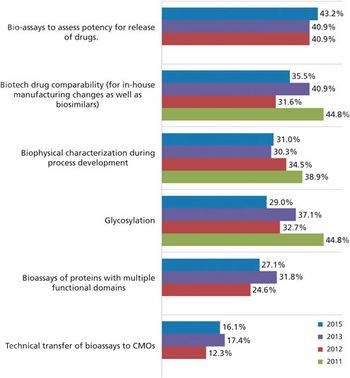
Biosimilars may be the key to CMO growth.

The company expanded its commercial packaging facility in response to a growing demand for pediatric drugs.

Avecia is adding drug substance capacity at its Milford, MA manufacturing site.

The new Boston laboratory offers advanced analytical testing services.