
Are strategic partnerships in clinical research a model for CMC services?

Are strategic partnerships in clinical research a model for CMC services?

Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

The report highlights a need for greater third party certification to ensure GMP vigilance.

Vetter has ready-to-submit documentation for this service in Common Technical Document (CTD) formats for the US, Europe and Japan.

The facility, which includes state-of-the-art formulation, analytical and synthetic laboratories as well as a customer training center, will focus on bioavailability enhancement and oral dosage formulations.

The industry may not be ready for India and China as regulatory issues emerge.
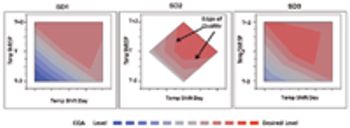
Traditional project decision-making vs. a QbD approach.
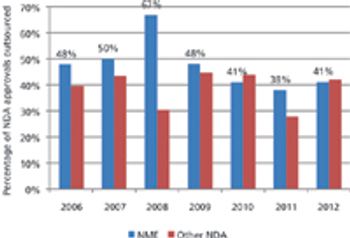
Approvals of new drugs are on an upward swing, but only a few CMOs are benefiting.

Outsourcing is weighing in more as a tactic for cost-cutting, but it is still not the primary weapon.

An innovative approach to capacity management.

Latin America's diverse growing market seeks regulatory harmonization.

CROs are keeping pace with the increased globalization of the biopharmaceutical/pharmaceutical industry.

Successful management of the CMO/client relationship should include open communication and trust.

A Q&A with SAFC and BioReliance about sourcing and risk mitigation for raw materials.

BioPlan's outsourcing survey gives insight on top activities, budgets, and growth trends in biopharmaceutical outsourcing.
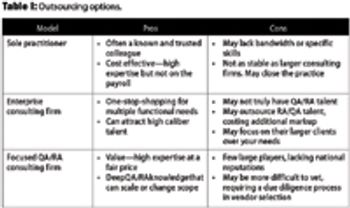
This article examines the options to best match needs and spending for quality and regulatory leadership.

The authors present solutions based on a review of current service offerings and their audit experience.

Growth is seen in outsourcing of insect- and plant-cell-based bioproduction expression systems.

Is the contract-only CMO an endangered species?
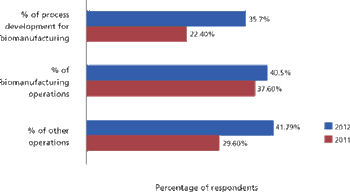
Will international biomanufacturing outsourcing become mainstream in this decade?

An introduction to a new series on manufacturing within global markets.

The procurement organization rethinks sourcing for maximum efficiency and results.

The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on mAbs.

Patrick Jackson of Vindon Scientific offers key considerations for choosing an outsourced sample storage facility.

The manufacturing capacity-sharing model between Merck and MedImmune ushers in a new paradigm of "co-opetition."