Mergers and acquisitions are positive for the CDMO industry, but there is a downside.

Mergers and acquisitions are positive for the CDMO industry, but there is a downside.

KBI Biopharma, a biopharmaceutical contract development and manufacturing organization, will manufacture an antibody and a fusion protein developed by Heat Biologics’ subsidiary.

Fresenius Kabi broke ground on a previously announced $250 million expansion of its Melrose Park, IL, manufacturing facility.

ADC Bio announces plans to expand into clinical and commercial drug manufacturing for ADCs.

Ajinomoto Althea opens manufacturing suites in new high potency and antibody drug conjugate commercial facility.

The acquisition adds to Catalent’s capabilities in biologics development, analytical services, manufacturing, and finished product supply.
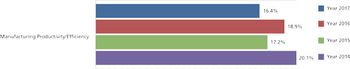
Innovation speeds discovery, drives down costs, and improves productivity.

Despite some progress, the industry is still in a wait-and-see mode regarding the administration, Congress, and FDA.

Catalent Applied Drug Delivery Institute announced a partnership with Rutgers University to examine the challenges of pediatric drug formulation and delivery.

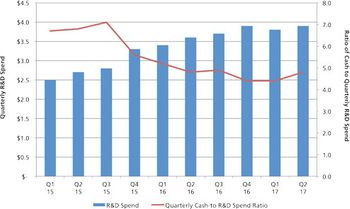
Contract manufacturers await a promising pipeline of drug products to jump-start stagnant growth.

The Bridgewater facility will provide a range of contract services for biopharmaceutical and pharmaceutical manufacturers, and also serve electronics chemicals suppliers.

Alcami will move its headquarters to Durham, NC while maintaining its manufacturing and laboratory operations in Wilmington, NC.

Catalent expands the scope of the OptiForm Solution Suite to bridge gap from late-stage discovery to Phase I trials.

The addition of 20,000 sq. ft. of manufacturing and office space increases Avecia’s Milford, Ma, oligonucleotide capacity to 3.0 mol.

GE Healthcare’s Dharmacon business and CordenPharma contract manufacturing enter a strategic collaboration to accelerate the oligonucleotide development process.

A $5.5 billion acquisition brings Capsugel’s oral dosage delivery capabilities to Lonza’s portfolio.

Samsung BioLogics signs $55.5 million agreement to manufacture tildrakizumab for Sun Pharma.

Patheon will add spray drying, sterile manufacturing, and packaging capabilities to four facilities.

IDBS described benefits and best practices for laboratory electronic data systems.

How has the bio/pharmaceutical contract manufacturing industry evolved and changed over the years and what does the future hold?

Fagron Sterile Services has voluntarily recalled three lots of Succinylcholine Chloride 20mg/mL 5mL syringe to the hospital/clinic level.
The success of a truly integrated continuous processing platform relies on the collaborative efforts of upstream and downstream specialists.
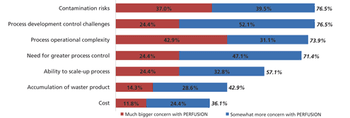
Although widespread adoption of continuous bioprocessing has been slow, some processes have been an exception.

Microdermics will focus on product development and clinical activities of new drug delivery methods, while Vetter’s primary role will be in the fill and finish aspect.

BioVectra will open its new microbial fermentation and complex chemistry site, including the capability to handle high-potency APIs, by the end of 2017.