
European CDMOs want into the US market, but entry options are limited.

European CDMOs want into the US market, but entry options are limited.

The trend of exits from the CMO industry looks to be gaining momentum.

A dynamic market, industry consolidation, and demand fluctuations lead to a mixed picture of pricing results.

A Q&A on bioanalytical method integrity with Roger Hayes of MPI Research.

Representatives of contract service organizations discuss technology trends with BioPharm International.

Annual study shows geographic proximity not a factor in CMO selection.

A well-constructed quality agreement can be an important tool to enable effective collaboration between owner and CMO.

The author highlights the top 10 outsourcing trends found during a survey of biopharmaceutical manufacturers.

As payers refuse to cover new drugs, CMOs take a hit.

Aesica Pharmaceuticals S.r.I. collaborates with QAD to meet China's Food and Drug Administration's shortened serialization deadline.
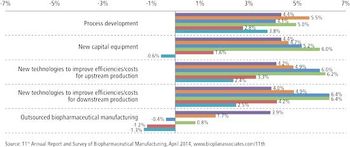
With budgets growing, clients see CMOs' costs as less crucial.

Evolving clinical trial research services give biopharmaceutical companies options for full- and functional-services.

Biopharma companies should not overlook India's growing market.

The CMO industry's value proposition is limiting its market penetration.

Outsourcing activity remains strong and unlikely to abate, especially in more traditional areas.

Vetter launches new serialization process to support track-and-trace efforts.

Changes in company ownership shake up the CMO industry.

Thirteen companies are accepted for participation in the supply chain program.

CMOs may find opportunities in alternative expression services.

With numerous biologics set to come off patent soon and the percentage of new therapeutics based on biomolecules growing, demand for contract manufacturing in the biopharma space is heating up.

The R&D model is in transition and creating new demands on contract services providers.

Partnering with CDMOs can help bridge the innovation gap.

Ongoing changes create new opportunities for CROs and CMOs.

Almac releases an enhanced third-party logistics customer billing application.

Lyra Myers, associate director and value creation agent at Roche, has been elected DCAT president.