
Implementing quality by design makes the determination of quality metrics across CMOs and sponsors essential.

Implementing quality by design makes the determination of quality metrics across CMOs and sponsors essential.

The contract provider needs to know as much as the NDA holder.

Formulators and developers are at the heart of the industry's basic premise-they are saving lives.

Industry experts discuss significant achievements. Plus: What's in store for the future.

Service providers must focus on delivering a superior customer experience.

Future sponsor-contract provider relationships will require more integration.

No matter the time or cost, knowing what's going on inside your facilities is always going to be worth the effort.

A closer look at elastomer changeout times provides one example of using industry knowledge to improve operations and cost.

Recovery audits and other best practices in procurement can improve the bottom line.

Collaboration can begin with a conversation.

Representatives from leading CROs weigh in on key challenges tied to the biopharmaceutical R&D, including issues regarding bioequivalence, platform technologies, process analytics, and more.

The author describes an equation that can be used to define the Quality relationship between a contract manufacturing organization and a client, including how to factor in both party's needs and regulatory commitments.
An architectural wonder teaches the biopharmaceutical industry a valuable lesson in project management.
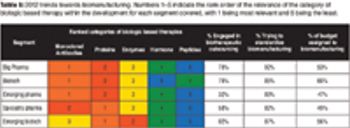
Growth in infrastructure and process-development capabilities will be required to support the biologics market.

Outsourcing in the pharmaceutical industry, until recently, has been largely confined to commercial manufacturing, packaging, and support for clinical trials.
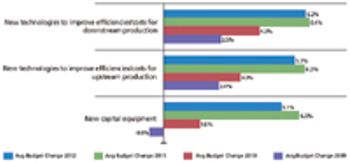
New outsourcing industry sectors have become multibillion-dollar industries.

Because of the complexity of the manufacturing processes for biologics, transfer of these processes to a contract manufacturer presents challenges.

The evolving bio/pharmaceutical business model poses risk for CMOs.

Stephen Taylor PhD, vice-president and commercial director at Fujifilm Diosynth Biotechnologies, addresses some of the challenges facing biologics outsourcing.
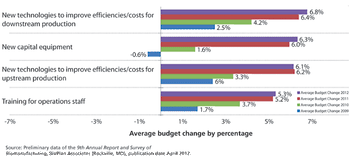
Industry optimism is on the rise for 2012.

China's healthcare reforms generate uncertainty for its domestic pharmaceutical market.

The census reveals the state of the population of India's health and the potential for growth in the healthcare market.

Industry announces plans for year ahead at annual JPMorgan Global Healthcare Conference.

The difficulties of doing business in China are offset by its adoption of more stringent GMP standards.

Contract organizations must have highly organized teams and plans to accommodate today's audits.