
Quality-risk management tools can assist biopharma companies in mitigating risks when outsourcing crucial elements of the drug-development process.

Quality-risk management tools can assist biopharma companies in mitigating risks when outsourcing crucial elements of the drug-development process.

Automation and disposables continue to reduce human error.

Contract service providers share insights on biopharma market developments and the implications of biosimilar drug approvals.

Whether outsourcing or developing cell therapies in-house, success demands a focus on quality, cost of goods, and sustainability from the start.

Agency officials visit China to meet with Chinese regulators and industry representatives about keeping the pharma supply chain safe.
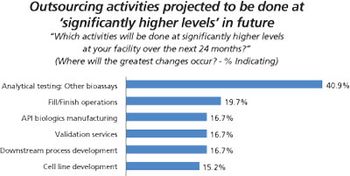
Biopharma companies on both sides of the Atlantic ship more of their assay testing to outside service providers.

While the United States and Europe still dominate, CMOs and CROs based in emerging markets continue to capture market share.

The rapid testing of biologic raw materials can lead to greater efficiency.

Baxter voluntarily recalls select lots of IV solutions due to possible particulate matter.

Cornell Stamoran, Catalent Pharma Solutions vice-president, is elected to the board of trustees of the Pharma & Biopharma Outsourcing Association.
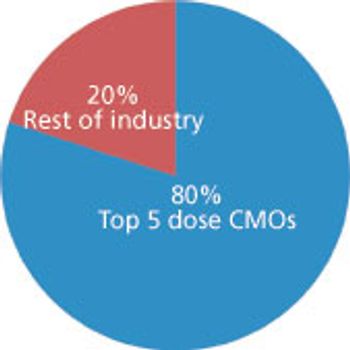
Big service providers get bigger faster thanks to Big Pharma.

As an affiliate member, TraceLink brings serialization expertise to the Pharma & Biopharma Outsourcing Association.

Rentschler Biotechnologie launches 2000-L single-use bioreactor and announces additional expansion.

The agency outlines recommendations for the development and submission of near infrared analytical procedures.

Patheon cites expanded API services with acquisition of IRIX Pharmaceuticals.
It is important to understand degradation and processing to maintain product stability in biologics.

Is there enough talent to go around?

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.

The fast growth of the global biopharmaceutical market has prompted global pharmaceutical and biotechnology companies to increase their R&D investment in biologics.

The draft guidance contains policies for drugs that are processed with additional manufacturing steps such as remixing, dilution, and repackaging.

The agency releases five draft guidance documents related to drug compounding and repackaging.

The new center represents AmerisourceBergen’s first facility erected specifically for clinical trial and commercial third-party logistics activities.

Market forces may limit the success of CMOs.

Nelson Patterson has been elected to Pharma & Biopharma Outsourcing Association board.
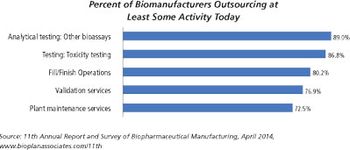
There are significant differences between small molecules and biologics fill/finish capacity.