
Optimize practices and meet requirements using electronic data integrity systems.

Optimize practices and meet requirements using electronic data integrity systems.
Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.

Sartorius Stedim Biotech expanded its range of single-use membrane chromatography solutions with Sartobind Cassettes.

The company added new products to its InfinityLab LC Series which will be showcased at HPLC 2017 in Prague.
The success of a truly integrated continuous processing platform relies on the collaborative efforts of upstream and downstream specialists.

Increased understanding of potential impurities has spurred efforts to standardize monitoring procedures.

Can bioprocessing runs be consistently replicated in an inherently variable production environment?
The unique structures of fusion proteins lead to expression, heterogeneity, and stability issues.

Continuous downstream bioprocessing is proving its worth, but connecting different operations and integrating upstream remains a challenge.
For cellular materials, new ultra scale-down devices inform large-scale downstream processing techniques.

Chromatography modeling can enhance bioprocessing efficiencies.
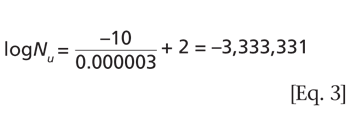
Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

The decision to use disposable bioreactors is now driven by commercial rather than technological considerations.
Although Protein A remains a top technology for monoclonal antibody purification, the industry continues to look for new approaches to improve conventional capture chromatography.

Pump systems must be designed to meet the needs of specific processes, including preventing cross-contamination and damage due to shear forces.

The membrane-based Protein A purification tool was unveiled at the 2017 PepTalk Conference in San Diego, California.

Excipient selection strongly influences lyophilization performance for biologic drugs.
There is much work to do to achieve efficient, cost-effective production processes.

A novel coiled flow inversion reactor (CFIR) improves process productivity and performance.
In addition to having the optimal cell line and process, it is crucial to have the optimal cell culture medium and feed to maximize performance potential.
Advances in technology are increasing the productivity and efficiency of commercial-scale chromatography bioprocesses.

A new virus-retentive membrane may be used to filter chemical-defined cell culture media for risk mitigation.
Improvements to aseptic manufacturing procedures are long overdue. But how feasible is it for manufacturers to modernize fill lines of legacy products?

Industry experts discuss best practices for selecting a separation technology.

Advances in cell culture media technology have helped achieve safer biologics.