
The $21.4-billion acquisition will create stand-alone business within Danaher’s life-sciences portfolio.

The $21.4-billion acquisition will create stand-alone business within Danaher’s life-sciences portfolio.
New ligands are being developed to meet the separation and purification needs of next-gen biologics.

A key technology that can help achieve a continuous production flow is single-pass tangential flow filtration.

The investment builds on a collaboration the companies entered into in 2007 for various biomanufacturing projects.

GE’s new facility, which will be operational in 2019, will produce a fiber-based chromatography platform for more efficient biopharmaceutical purification.

Fujifilm Diosynth Biotechnologies and the Centre for Process Innovation (CPI) are collaborating to complete the technology transfer of the expression and purification of model monoclonal antibodies (mAbs) as part of AMECRYS, a research project funded by the European Commission.

Survey results and record attendance may show positive signs for established and emerging biopharma regions.
The second half of this article describes the testing methods used by the authors to demonstrate the applicability of single-use mixing technology for virus inactivation.

The companies partnered to build a 500-L single-use pilot-scale plant for biologics production.

The maturation of single-use technologies presents commercial bioprocessing options for small-volume drug products.
This article explores the use of single-use mixing technology in a detergent-based virus inactivation step during a monoclonal antibody production process.
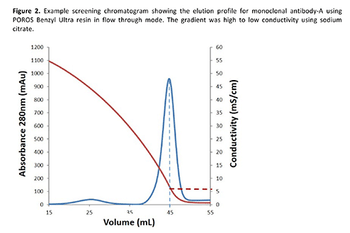
Hydrophobic interaction chromatography (HIC) in flow-through mode offers a more efficient and cost-effective polishing/purification process to remove monoclonal antibody aggregates while maintaining purity at ≥99% than a mixed-mode bind/elute procedure.
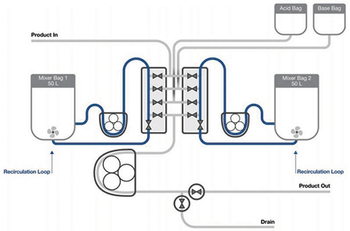
Testing demonstrates an automated semi-continuous process strategy for viral inactivation with steps that mimic batch processing.

The provider of plant-based ingredients will present recently launched multi-compendial materials for upstream and downstream biopharmaceutical applications.

Bio-Rad introduces CHT Ceramic Hydroxyapatite XT media and Nuvia HP-Q resin resin for process protein purification.

New products were developed as next-generation process intensification technologies, MilliporeSigma reports.

Sharing of bioprocessing know-how can help resolve pressing industry problems.

Automation can improve many aspects of bioprocessing, but several hurdles must be overcome before the full range of benefits can be realized.

More complex biologic samples must be evaluated to ever higher levels of specificity and sensitivity.

Biopharma companies can balance competing demands from patients, investors, and regulators by keeping a focus on science.

Increasing demand for biologics is driving the need for innovation in bioprocessing.

The new resin used a combination of “jetting” technology and a high-performance Protein A ligand.

The company’s next-generation ultraperformance liquid chromatography platform is designed to meet the evolving laboratory requirements for chromatographic performance.

Higher cell titers and cell densities have posed a challenge to cell harvesting, a crucial step in biologics manufacturing.
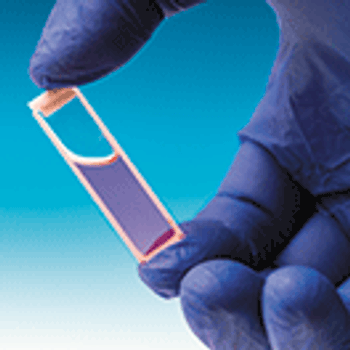
The National Institute of Standards and Technology (NIST) has developed Standard Reference Material (SRM) 2082 as a pathlength standard for UV absorbance measurements for use with the new generation of microvolume spectrophotometers and short-pathlength cuvettes.