
The authors investigate the sufficiency of high-temperature short-time treatment in inactivate mouse minute virus contamination.
Raymond W. Nims, PhD, is a biosafety consultant at RMC Pharmaceutical Solutions, Inc.

The authors investigate the sufficiency of high-temperature short-time treatment in inactivate mouse minute virus contamination.
The authors present a case study identifying a contaminant.
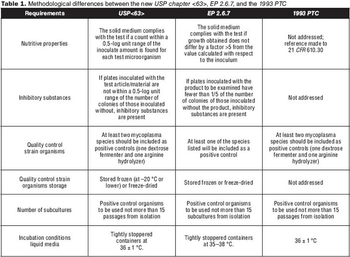
What you need to know about USP chapter <63>.
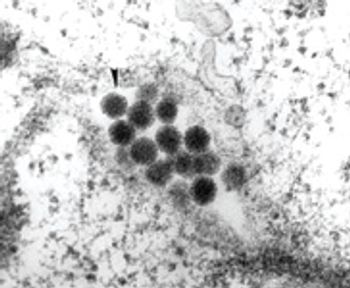
Avoid manufacturing failures by effective viral inactivation.
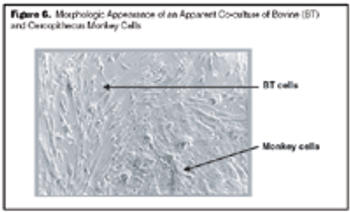
Strategies for accurate speciation and case studies for the detection of cell line cross-contamination using a commercial kit.
Published: June 1st 2011 | Updated:
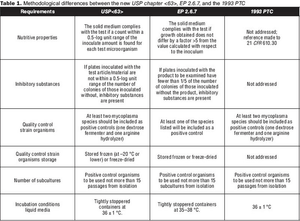
Published: August 1st 2010 | Updated:
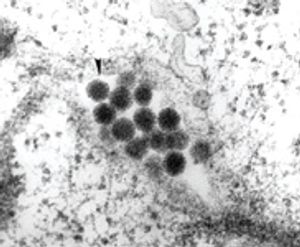
Published: October 1st 2008 | Updated:
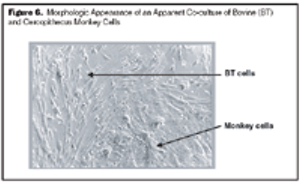
Published: June 1st 2005 | Updated:

Published: April 1st 2016 | Updated: