
Technologies in use and on the horizon could change aseptic processing in ways that seemed inconceivable years ago but approaches to aseptic process validation still need to move beyond their 1970s roots.
James P. Agalloco is the president of Agalloco & Associates, P.O. Box 899, Belle Mead, NJ 08502, tel. 908.874.7558, jagalloco@aol.com. He is also a member of Pharmaceutical Technology’s editorial advisory board.

Technologies in use and on the horizon could change aseptic processing in ways that seemed inconceivable years ago but approaches to aseptic process validation still need to move beyond their 1970s roots.

Risk aversion is impeding the use of closed aseptic systems, and adding unnecessary cost and complexity to aseptic operations in the pharma industry.

Questions and answers about contemporary closed-system design criteria for aseptic processing equipment

The industry’s aversion to risk has led to its treating closed aseptic processing systems as miniature cleanrooms, resulting in redundant and expensive practices. This article ponders this impasse, examining new technologies and applications in light of past regulatory guidance and more than 40 years of operating evidence.

Alternatives to time-consuming, error-prone operations promise to reduce vaccine manufacturing costs and improve facility flexibility.
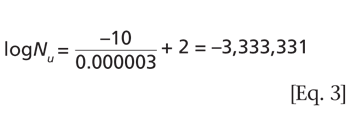
Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.
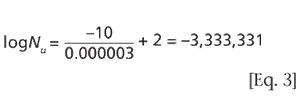
Published: April 1st 2017 | Updated:

Published: November 15th 2017 | Updated:

Published: May 1st 2020 | Updated:

Published: May 12th 2020 | Updated:

Published: May 12th 2020 | Updated:

Published: August 1st 2020 | Updated: