
India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.
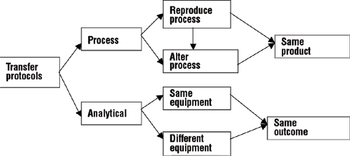
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.

How an electronics engineer led the first Indian company to carry out indigenous development of a recombinant vaccine.
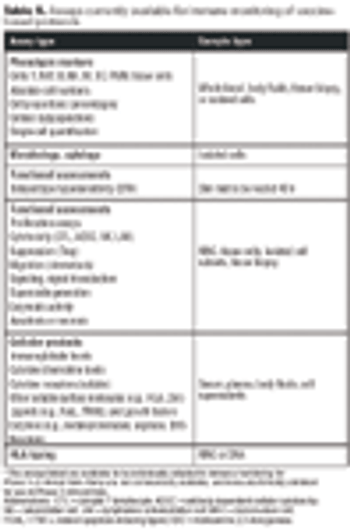
Emerging therapies pose challenges for standardizing QC.

Need a standard to follow? Just ask, What would Genentech do?

Genentech has been notified that their Cabilly patent claim has been rejected by the US Patent and Trademark Office.

Two case studies illustrate a systematic approach.

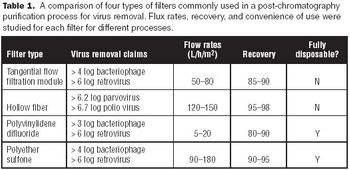
The use of disposables has changed significantly in the biopharmaceutical industry.

Best practices from Big Biotech, including how to handle new product introductions.
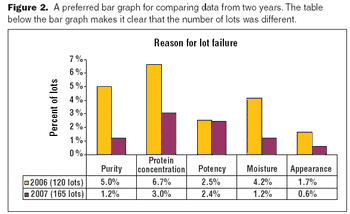
The key to a good graphical presentation is to select the method that best fits the data.

The automated NucleoCounter from New Brunswick Scientific use disposable cassettes, pre-filled with propidium iodide for accurately counting cells.

The replacement of a IgG ELISA assay with an SPR-based test can shorten the production workflow by one day.
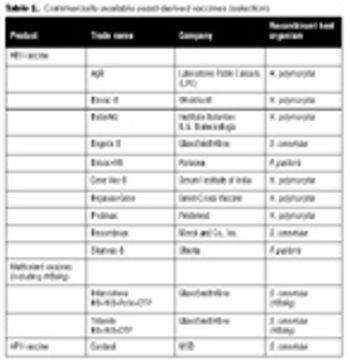
The recombinant approach lends itself to the production of an inexpensive and effective vaccine at large scale.

Potential interference with maternally-derived antibodies makes most vaccines less effective in the neonate.
Human HBV can be grouped into eight genotypes, which differ by at least 8%.
Microstructured transdermal systems can deliver a vaccine in close proximity to the antigen-presenting cells in the epidermis.

A solution for the problems of a "bag in a can" system would be a fully jacketed and insulated container, similar to a traditional freeze tank.
One of the challenges of adopting single-use technology is that not all cell lines are compatible with disposable bioreactors.
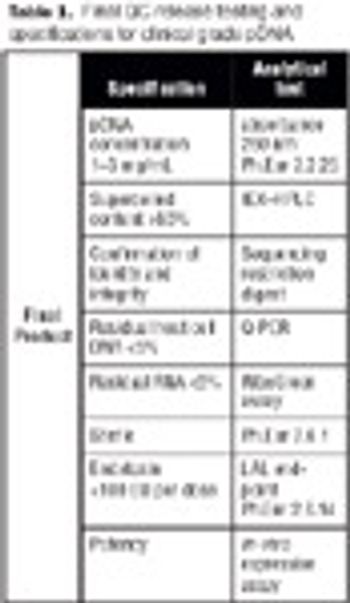
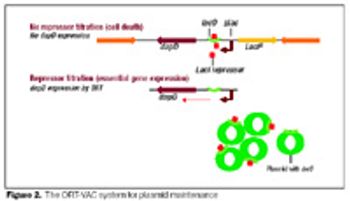
Disposables are increasingly being used in the manufacture of biopharmaceuticals. This article describes the design of a fully disposable process for the cGMP manufacture of clinical trial grade plasmid DNA. It addresses the rationale for implementing such a process with respect to the manufacture of patient-specific plasmid DNA vaccines for the treatment of leukemia. The process incorporates a number of disposable technologies, which are simple to use and thus reduce the need for investment in expensive equipment and cleaning validation.
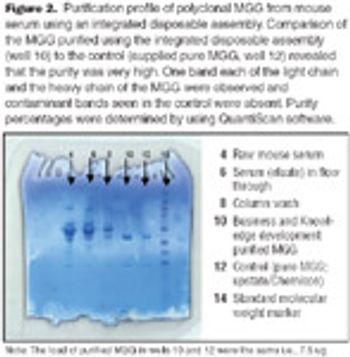
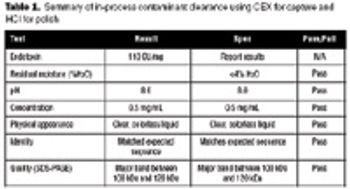
Comparison of the integrated assembly purified MGG to the control revealed that the purity if the MGG was very high.
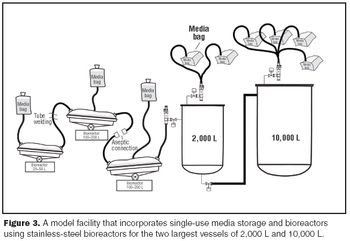
Single-use systems reduce maintenance and capital expense by eliminating expensive vessels, valves, and sanitary piping assemblies.


The approval of a new seasonal influenza vaccine has further diversified the supply to the US market.

The US Food and Drug Administration has approved the use of the nasal influenza vaccine FluMist in children ages 2?5.