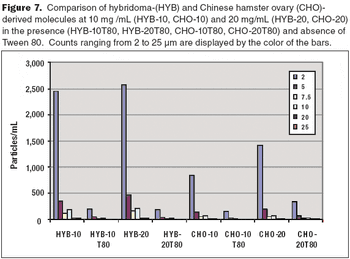
How to maintain product stability and prevent particulates.

How to maintain product stability and prevent particulates.

What end users think about single-use systems.

Members of the pharmaceutical and biotech industries have formed a new industry coalition, called "Rx-360," with the goal of improving supply chain safety.

This article reviews some of the commonly used approaches for process monitoring as well as the evolution of process monitoring in the Quality by Design (QbD) paradigm.
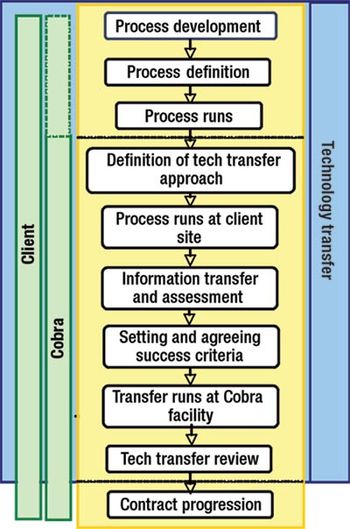
Effective tech transfer can save time and effort in later manufacturing processes.

Biodefense start-up companies have an abundance of options when seeking funding.
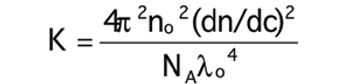
Can increase in ionic strength result in higher viscosity?
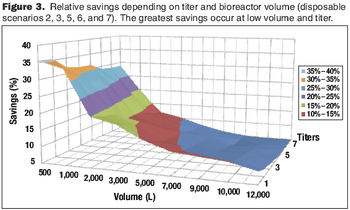
Cost modeling provides valuable insights to support strategic decision-making when implementing disposable technologies.

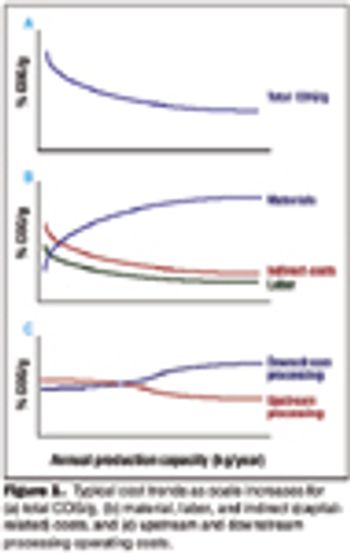
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

Altering the order of operations, using new resins, and increasing dynamic binding capacity can obviate the need for major facilty changes.

A purification scheme to maximize the efficiency of the purification process and product purity while minimizing the development time for early-phase therapeutic antibodies.
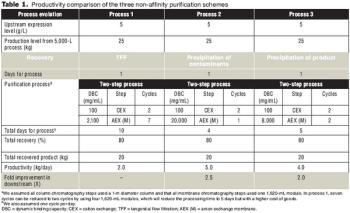
In three non-affinity purification processes based on cation exchange capture with high binding capacity, applying a host cell protein exclusion strategy enabled robust scale up and better economics.
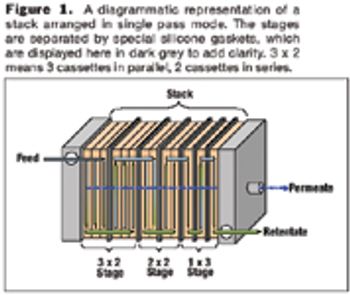
New techniques can greatly improve the MAb purification process.
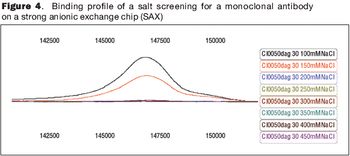
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

A close-up look at Pfizer's biotherapeutics plant in Shanbally, Ireland.

With counterfeit and adulterated medicines posing an increasing risk to patients in the United States and worldwide, the US Pharmacopeial (USP, Rockville, MD) Convention announced on February 4, new standards for two widely used drug products that have been involved in episodes of adulteration resulting in patient deaths.

The US Food and Drug Administration has launched a voluntary pilot program that will help promote the safety of drugs and active drug ingredients produced outside the United States.

How to choose a disposable mixing system that fits your particular needs.
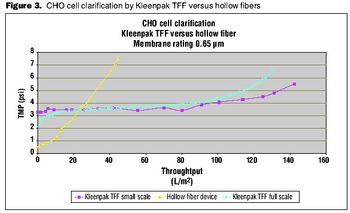
Choosing the right tools to enhance the process.
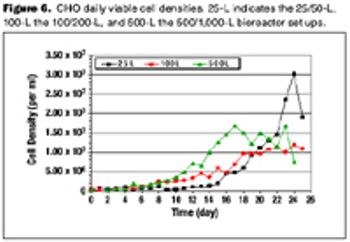
Are disposable bioreactors effective for cell culture?
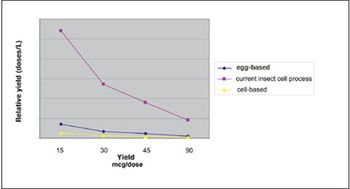
With virus-based production, vaccines can be available in 10-12 weeks.
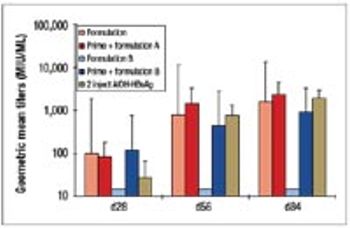
The disadvantages of the traditional vaccine regime (prime plus boost) have spurred the development of single-shot vaccines. This article describes the development and manufacture of a prototype single-shot vaccine that uses microspheres made from cross-linked modified dextran polymers for controlled release of the antigen.
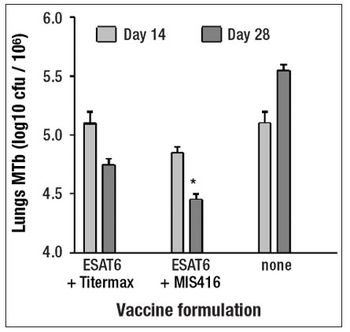
This article discusses the potential of MIS416 adjuvant, a vaccine adjuvant and immunogen co-delivery system, to provide adequate immunostimulation to overcome host factors that may limit the success of therapeutic vaccines.

Understanding the relationship between the process and CQAs.