
Roche (Basel, Switzerland, www.roche.com) has opened its new biotechnology production center in Basel.


Roche (Basel, Switzerland, www.roche.com) has opened its new biotechnology production center in Basel.

The FDA issued MedImmune, Inc. (Gaithersburg, MD, www.medimmune.com), a warning letter for violating the agency's manufacturing rules and held off approving the company's influenza vaccine for use in children younger than age five until the problems are resolved.

The US Food and Drug Administration (FDA, Rockville, MD, www.fda.gov) has issued final recommendations for increasing the supply of safe and effective influenza vaccines for both seasonal and pandemic use.

Intas Biopharmaceuticals Limited (IBPL, Gujarat, India, www.intasbiopharma.co.in) has become India's first dedicated biopharmaceutical company to receive certification from the European Agency for the Evaluation of Medicinal Products (EMEA, London, UK, www.emea.europa.eu) or an EU-GMP certification for its manufacturing facility at Ahmedabad, Gujarat.

The BioWelder and BioSealer from Sartorius (www.sartorius.com) are suitable for connecting or disconnecting thermoplastic tubing in biopharmaceutical manufacturing processes.

Today, most disposables are used for process development and clinical-scale manufacturing. Substantial growth in disposables usage may not occur until disposables are incorporated into the production of licensed products at commercial scale.

Discrete simulation allows the design teams to see every meaningful detail about equipment and materials flowing through the process.

Eden Biodesign (Liverpool, UK, www.edenbiodesign.com), SAFC (St. Louis, MO, www.sigmaaldrich.com/SAFC/Pharma), Midatech Group (Oxfordshire, OX, www.midatechgroup.com), Cellexus Systems (Cambridgeshire, UK, www.cellexusbiosystems.com), and BioConvergence LLC (Bloomington, IN, www.bioc.us) are sprucing up their product development and services with the construction of new manufacturing facilities.

Millipore Corporation's disposable mixer is designed to help companies mix pharmaceutical ingredients and prepare cell culture media.

Energy input is affected by rocking the chamber back and forth, generating a fluid movement in the cell culture and medium.

This flexible setup minimizes the number of purification process steps, buffers, and process components.

Membrane-based chromatography technologies sometimes offer advantages over resin-based technologies.

Fortunately for all of us, not everyone's head is in the sand.

The FDA's (Rockville, MD, www.fda.gov) first approval in the United States of a vaccine for humans against the H5N1 influenza virus marks an important step forward in protecting the public against a pandemic influenza outbreak.

Abbott (Abbott Park, IL, www.abbott.com) recently announced the official opening of its new biologics manufacturing facility in Puerto Rico to support the long-term supply of its leading biologic agent, HUMIRA (adalimumab), and other future biologics.

Singapore's efforts to grow its biologics manufacturing sector received a significant boost on March 28, 2007, when the Singapore Economic Development Board (EDB) announced that Genentech, Inc. (San Francisco, CA, www.gene.com) has decided to establish a commercial-scale microbial-based biologics manufacturing facility in Singapore.

Process monitoring ensures that the process performs within the defined acceptable variability that served as the basis for the filed design space.
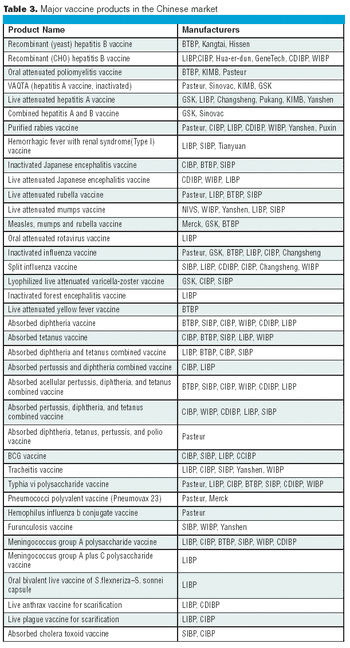
The Chinese vaccine market competition is now transferring from the former price-competition model to a technology- competition model.
Frequently asked questions on implementing and using single-use technologies

Scale-up issues leading to long development times and deviations in the commercial facility is a critical challenge.

Although contaminants and other parameters may be main causes of filter breakdown, some nanofilters still remove viruses at high Log Reduction Value (LRV).

The biopharmaceutical industry has gained a lot of experience in monitoring glycosylation, but still has a lot to learn about the structure–function relationship.

The United States Pharmacopeia (USP, Rockville, MD, www.usp.org) and the UK's National Institute for Biological Standards and Control (NIBSC, Hertfordshire, UK, www.nibsc.ac.uk) are seeking participants in a study of analytical methods used by the industry to characterize and quantify oligosaccharides.

Sartorius AG (Goettingen, Germany, www.sartorius.com) has acquired a substantial stake in the biopharmaceutical supplier Stedim Biosystems S.A (Aubagne, France, www.stedim.com) and combined its biotechnology division with Stedim.
To shorten time to market for new therapeutic proteins, new and fast methods, such as high throughput screening, are needed to speed up downstream processing. The platform technology discussed in this article includes a structural approach that can be used as a general procedure to purify therapeutic proteins. The approach starts with ligand screening and selection-on-a-chip, with the Surface Enhanced Laser Desorption Ionization–Time of Flight (SELDI–TOF) mass spectrometer system. Next, resin screening and supplier selection are performed using robotics, followed by scouting studies under dynamic conditions to select the best resin. Finally, optimization studies of critical parameters are carried out with statistical design approaches (design of experiments). A few examples are presented to explain the platform approach for purification development in more detail.