
Manufacturers of biopharmaceuticals can improve productivity by taking patient wellness into account.


Manufacturers of biopharmaceuticals can improve productivity by taking patient wellness into account.
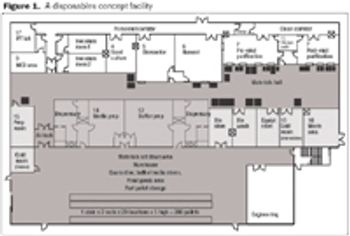
In addition to making technical developments, vendors are also looking at ways to improve supply-chain security. By offering standard, off-the-shelf products, vendors are able to shorten lead times and improve the security of supply.

As the use of disposable bioprocessing equipment has increased, a new question is gaining prominence: What is the best way to dispose of the equipment after use?

SciLog, Inc. (Middleton, WI), a privately held company that designs and manufactures computer-controlled bioprocessing equipment, has announced the signing of a patent licensing agreement with GE Healthcare (Somerset, NJ).
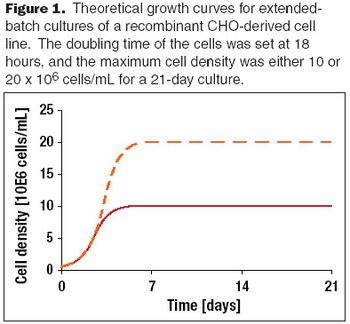
A discussion of past achievements and future expectations of recombinant protein production yields from mammalian cells.

With a variety of recombinant, animal-free, defined protein supplements such as growth factors, transferrin, and albumin entering the market, the biopharmaceutical industry now has innovative and safer alternatives to serum and other animal-derived supplements.
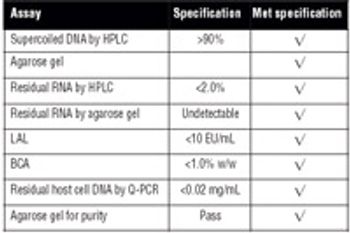
Recombinant protein and plasmid DNA production using microbial expression systems is the cornerstone of many biologics manufacturing processes. HCD methods are commonly used for these processes because of the advantages they provide.
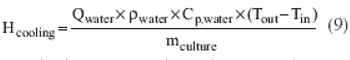
Process-modeling tools can ensure smooth tech transfer.
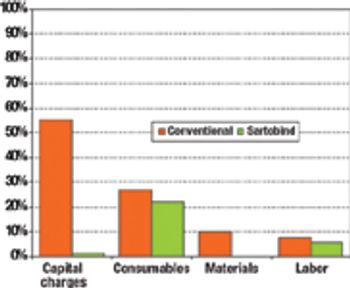
The current focus on cost-of-goods (COGS) models is underplaying the benefits of disposables technology in biopharmaceutical manufacturing. The best method for accounting for the benefits of reduced and delayed capital expenditures is through the use of NPV analysis.

It is important to understand critical aspects of the CMO's capabilities. Only by auditing certain key areas can the sponsor be assured of the quality of the materials produced.

The industry needs to open up to validation failures.

US Food and Drug Administration's Division of Biologic Oncology Products has approved two new biologics license application (BLA) supplements expanding the approval of Genentech's Herceptin (trastuzumab) for the treatment of breast cancer.

DSM Biologics (Heerlen, the Netherlands), and Upfront Chromatography A/S (Copenhagen, Denmark) have announced a collaboration to optimize Upfront’s new, fully disposable chromatography system for use with DSM's proprietary manufacturing technology.

The FDA is under attack from all sides. Many influential members of Congress either don't trust the agency to monitor the industry appropriately, or have found it politically expedient to keep sounding alarms about inadequate oversight of food and drug safety and clinical research. The good news is that there seems to be a growing consensus that FDA needs a major infusion of cash to regain its stature as an effective science-based regulatory agency.
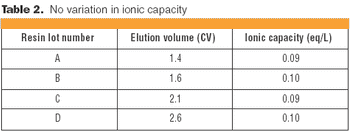
Understanding the impact on process performance.

In the context of process validation, the confirmation of a belief must be checked repeatedly, throughout the product lifecycle.
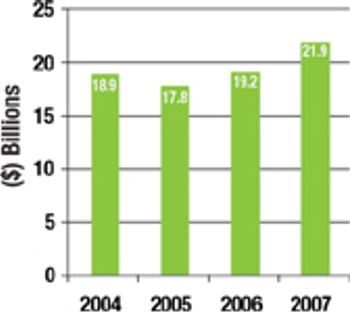
The current overcapacity situation in the bio/pharmaceutical industry is a reminder that CMOs need to come up with business models and value propositions that are based on more than just selling capacity.
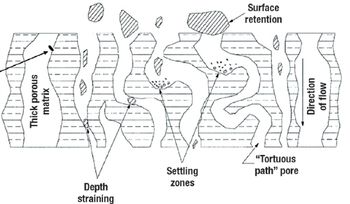
A comparison of primary harvest techniques.

Recent problems with food and pharmaceutical ingredients sourced from China highlight a major disadvantage of our complex international supply chains for food and drug ingredients. A global supply chain offers more opportunities for accidental contamination as well as intentional adulteration and counterfeiting. Sticking to minimal requirements may not be enough.

The book is a useful, comprehensive, and truly an excellent reference source of biopharmaceutical information.

Disposable technologies that mimic the conventional stainless-steel bioreactor will be most readily adopted

A case study investigated the root cause of failures in sterile filtration by evaluating the effects and interactions of four operating parameters.

The heparin safety crisis puts a spotlight on manufacturing processes and regulatory oversight.

Now is a good time for companies to know their suppliers well.

Saint-Gobain Performance Plastics Corporation (Aurora, OH) has acquired the assets of J & J Scientific Products, Inc. (Tampa, FL), a manufacturer of disposable plastic products for the biopharmaceutical market.