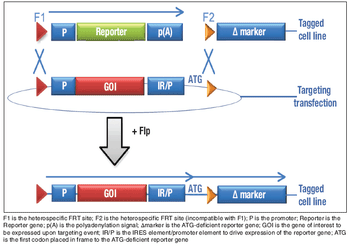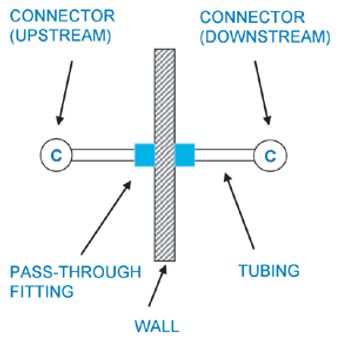
Many factors must be considered when choosing a sterile connector for a given process.

Many factors must be considered when choosing a sterile connector for a given process.
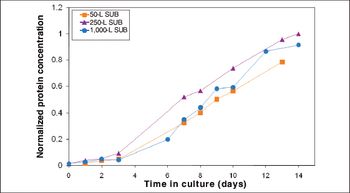
Process performance was comparable across all scales, and fiber optic sensors appeared interchangeable with conventional probes.
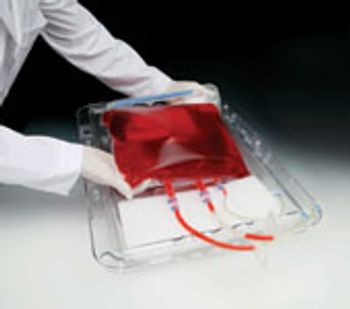
Genentech's evaluation of single-use technologies for bulk freeze-thaw, storage, and transportation.

An enterprise-wide quality management initiative is required to maintain supplier quality without sacrificing bottom-line objectives.
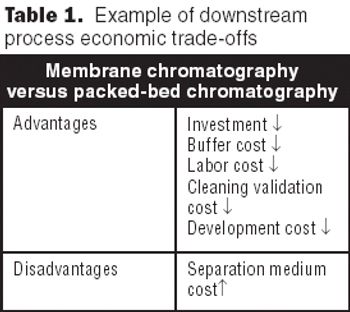
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

New techniques can overcome bottlenecks in existing facilities.
A purification scheme to maximize the efficiency of the purification process and product purity while minimizing the development time for early-phase therapeutic antibodies.

The industry and government must collaborate to develop robust technologies and quicker, more flexible manufacturing approaches for vaccine development.
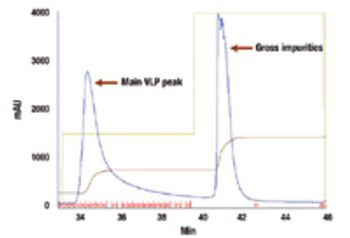
New technologies such as virus-like particles are promising weapons in the battle against pandemic influenza.

Membrane-based TFF technology can ease scale-up and provide a higher recovery percentage compared to conventional purification methods.
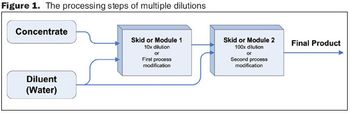
Automated in-line dilution can help solve capacity, financial, and quality concerns that biopharmaceutical manufacturing plants may be facing.

It is now possible to combine antigens with specific adjuvant systems to create more effective vaccines.

Small changes can have a big effect further downstream in your manufacturing processes.
Needle-free vaccine delivery platforms can solve the problems of stockpiling, cold-chain management, and pandemic preparedness.

To assess current trends in single-use bioprocessing equipment, BioPharm International turned to John Boehm, bioprocessing business unit manager, Colder Products Company; Mandar Dixit, head of product management?filtration technologies, Sartorius Stedim Biotech; Geoff Hodge, managing director of process technology Xcellerex, Inc.; Günter Jagschies, senior director of strategic customer relations, life sciences, biotechnologies, GE Healthcare; Mani Krishnan, director of product management, Mobius single-use technologies, Millipore Corporation; and Jerry Martin, senior vice president of scientific affairs, Pall Life Sciences, and board chairman and technology chair at BPSA.

To assess current trends in laboratory equipment, BioPharm International turned to Francis Bach, North American sales director, Asahi Kasei TechniKrom, Inc.; Erika Lapinskas, PhD, product manager, Sartorius Stedim Biotech; Jeffrey R. Mazzeo, PhD, biopharmaceutical business director, Waters Corporation; Amber Ratcliff, product manager, analytical sensors, Hamilton Company; and Josh Silverstone, marketing director, Millipore Corp.

To assess current trends in information technology, automation, and process control, BioPharm International turned to Rick E. Cooley, market development manager?process analytics, Dionex Corporation, and James Erickson, president and chief executive officer, Blue Mountain Quality Resources, Inc.

To assess current trends in cleanrooms and engineering & facilities, BioPharm International turned to Parrish Galliher, founder and chief technology officer, Xcellerex, Inc.; Jim Maslowski, owner, PDC Aseptic Filling Systems; Morgan Polen, vice president, application technology, Lighthouse Worldwide Solutions; and Benoît Verjans, commercial director, Aseptic Technologies.
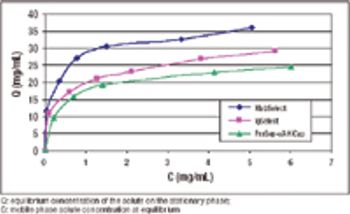
A stable alternative to Protein A chromatography.

The House Energy and Commerce Committee approved a legislative amendment that would give 12-years data exclusivity to innovator biologics. The amendment was introduced by Reps. Anna Eshoo (D-CA) and Jay Inslee (D-DC).

Single-use technologies can be configured and installed fairly quickly, but are they ready to handle the urgency and scale of a pandemic?

Enabling site-wide process efficiency.

The Senate?s Health, Education, Labor, and Pensions Committee yesterday passed The Affordable Health Choices Act, the committee?s healthcare reform legislation that gives 12 years of data exclusivity to innovator biologics. The committee had adopted the 12-year data protection amendment to the healthcare legislation on Monday.

In advance of the Senate Health, Education, Labor, and Pensions Committee?s meeting to be held on Friday to consider amendments to the healthcare reform bill, including several amendments related to biosimilars, Biotechnology Industry Organization?s (BIO) President and CEO Jim Greenwood reaffirmed BIO?s support for a 12-year data exclusivity period for biologics.