
The supply chain can be used as a tool for managing not only product supply risks, but overall business risks as well.

The supply chain can be used as a tool for managing not only product supply risks, but overall business risks as well.

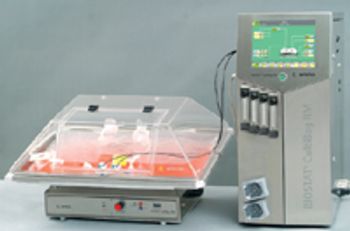
The disposable FibraStage cell culture system from New Brunswick Scientific (www.nbsc.com/b8.htm) is available in two models.

Invitrogen (San Diego, CA, www.invitrogen.com) believes that scaledown technologies can significantly improve clone selection and cell culture media development.

NovAseptic mixers from Millipore (www.millipore.com/bioprocess) are designed for a variety of mixing applications in the pharmaceutical and biotechnology industries and are magnetically driven, which minimizes contamination risk.
The many benefits of disposable technologies, such as significant savings in time, labor and capital, as well as ease of scalability and flexibility, have led to the growing trend of adopting disposable technologies in bioprocess manufacturing processes.
In recent years, disposable membrane chromatography has gained acceptance as a robust device for large-scale processing.
Biopharmaceutical processes typically require a significant investment in equipment-often a substantial obstacle for start-up companies. The risk of drug development failure is often high, further limiting access to the required capital. Flexibility and lower capital outlays are required not only by start-up companies, but also by research organizations with multiple product lines and by companies requiring quick capacity increases. Disposable technologies offer the highest potential for these companies to meet their business requirements. With lower capital requirements and increased flexibility, disposables are an important part of these companies' risk management strategy.
The adoption of single-use containers in the biopharmaceutical industry is becoming more frequent as the popularity and availability of the technologies increase. The choice of a solution for storage in single-use containers clearly depends on the application and the inherent risks associated with the application. A "one fits all" single-use system cannot respond to all the requirements of a particular step in a biopharmaceutical process, much less to all the steps of a process. The needs of an application will lead to very specific single-use solutions.

The deliberate reuse of disposables has caused many clinically significant problems.
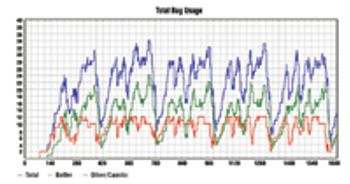
In its early days, the biotech industry was almost entirely science driven, but it has since expanded from a laboratory environment to a sophisticated and dynamic manufacturing environment. As technological discoveries are increasingly translated into commercial products, biotech companies are realizing that they must generate a stronger return on assets.

Filtration systems exemplify disposable technologies that can be presterilized.

Increased resin stability can extend the number of cleaning cycles that can be performed in situ.

The number of biotechnology-based human therapeutic products in the late-stage pipeline, and the average cost to commercialize a biotech product, have steadily increased. This has required biotech companies to use economic analysis as a tool during process development and for making decisions about process design. Process development efforts now aim to create processes that are economical, as well as optimal and robust.

If a company wants to reduce costs, it should consider outsourcing some manufacturing and analytical testing to low-cost sites.

Whether it's for manufacturing drugs, characterizing cell substrates, or regulating new technology, quality systems provide a needed framework.

We often assume we know what success looks like for our partner, but we never ask them, or take the time to write it down.
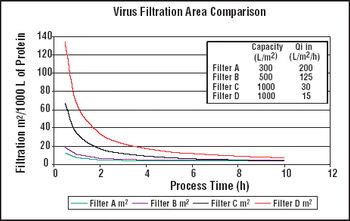
It is important to ensure that flow decay during processing is comparable to that observed during retention studies.

Formulations for pulmonary inhalation comprise spherical, porous particles that are 1–3 microns in diameter.

Established, fully validated methodology and SOPs are required prior to initiation of any training activities.

The challenge is to determine the optimal frequency for preventive maintenance and the optimal frequency and tolerances for calibration readings.

Steam traps are part of a steam-in-place system. The current design allots 18 in. of vertical leg for condensate backup. A design with a sensitive bellows has been proven in laboratory tests to need only 6 in. of vertical leg during the 15 min. of 121?C sterilization. Loads of 1 to 27 lb/h are covered by the capability of the new trap, equivalent to required steam for vessels 20 to 40,000 L.

Increasingly, pharmaceutical companies have recognized that software development is not their core competency.

Understand your company's requirements, define responsibilities,and manage your team effectively.
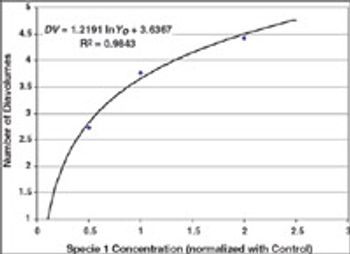
Case studies were run to test Process Analytical Technology applications for protein refolding, diafiltration, and cation exchange chromatography. It is shown that it is feasible to design control schemes that rely on measurement of product quality attributes and thereby enable real-time decisions.
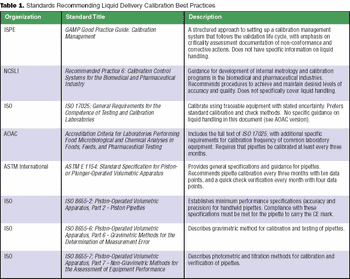
Federal regulations are broad and open to interpretation. Most have not caught up with advancements in technology.