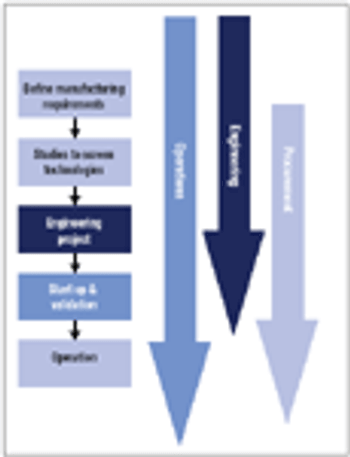
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.

In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.
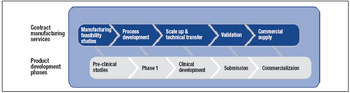
Four reasons why outsourcing may be the best option, and key factors to consider when selecting a provider.

No time for QbD? How to convince management to make it a priority.

The FDA's revised process validation guidance manages to explain the underlying concepts of Quality by Design without every using the phrase.
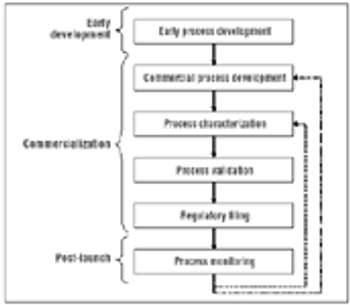
Using multivariate experiments to define acceptable ranges.
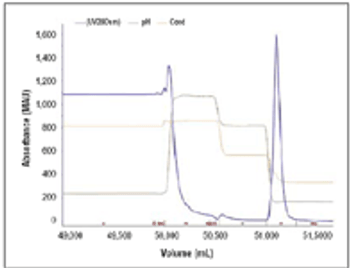
Results from a process developed for a commercial antibody.

A major problem in personalized medicine has been scale-up.

The European Medicines Agency (EMEA) has granted license approval to Bayer HealthCare LLC (Bayer, Berkeley, CA) for its new sterile filling facility on its Berkeley, CA, campus.

Millipore Corporation (Billerica, MA) and Applikon Biotechnology BV (Schiedam, The Netherlands) have announced an agreement to co-develop and distribute disposable bioreactor systems for biopharmaceutical applications.

Transitioning from stainless steel technology to disposable technology in bioprocessing.
This article describes the steps required to build a comprehensive model in a batch process simulator for a process that uses single-use systems for buffer preparation and storage.
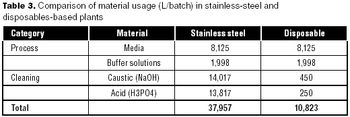
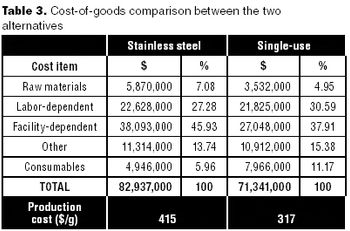
Can disposables reduce your facility's environmental footprint? We have compared the environmental footprint of a traditional biopharmaceutical manufacturing facility using fixed-in-place stainless steel equipment, and a facility implementing disposable technologies for cell culture, solution mixing and hold, product hold, and liquid transfer.

This article describes a quick method for evaluating lifecycle costs for single-use systems against their more conventional stainless-steel counterparts.
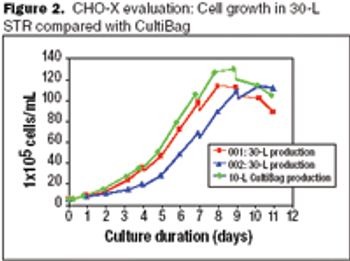
Productivities similar to those achieved with stirred tanks can be achieved with disposable bioreactors.

There could be a serious glut of commercial scale mammalian cell culture capacity over the next five years. Then again, there could be a significant shortage. It all depends on how things develop in expression technology, the new product pipeline, and corporate strategies.
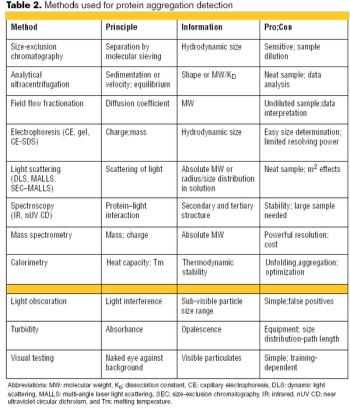
FDA perspectives on specs and effective control strategies.

The FDA and other regulatory authorities are evaluating new regulations to ensure the safety and quality of nanomaterials in biomedical products.

Despite creating plastic waste, the disposables option is better for the environment.

Interview with David Radspinner, director of global marketing & customer applications, bioprocess production at Thermo Fisher Scientific.

When making critical decisions such as whether to build or buy critical capabilities, companies need a decision-making approach that weighs risks and rewards as a science with adequate inputs, repeatable processes, and measurable results. The method must also accommodate the human factor by encouraging wide participation and providing the kind of neutral decision criteria that satisfies participants about the objectivity of the process.
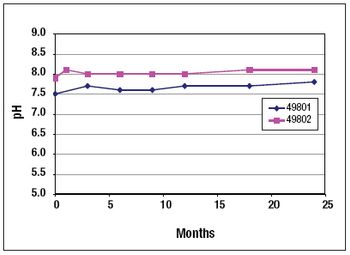
NeisVac-C is a polysaccharide-protein conjugate vaccine for use against Neisseria meningitidis serogroup C infection. The Phase 1 clinical formulation consisted of physiological saline, thimerosal, and aluminum hydroxide. The long-term stability data for both the PBS and saline formulations are presented in this article.

Interview with Roger Lias, president and group commercial director at Eden Biodesign, Inc.
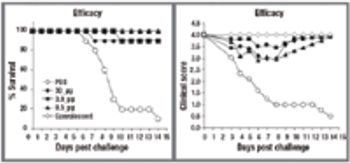
This article discusses the production process of the major influenza antigen, hemagglutinin (HA), by rDNA methods in E. coli.

Interview with Magda Marquet, co-CEO and co-president of Althea Technologies
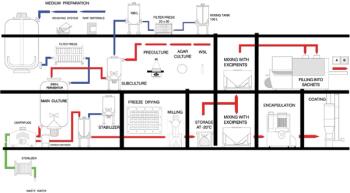
There are a number of specific characteristics to be considered when developing and manufacturing live bacterial vaccines.