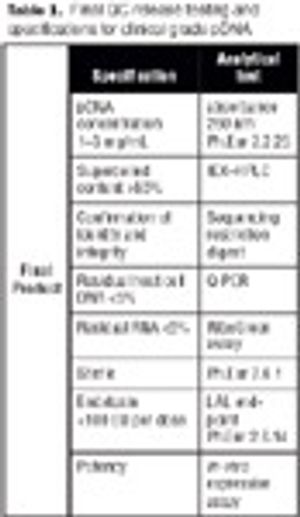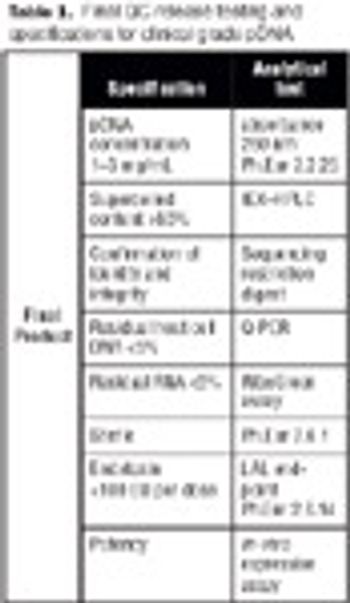
Disposables are increasingly being used in the manufacture of biopharmaceuticals. This article describes the design of a fully disposable process for the cGMP manufacture of clinical trial grade plasmid DNA. It addresses the rationale for implementing such a process with respect to the manufacture of patient-specific plasmid DNA vaccines for the treatment of leukemia. The process incorporates a number of disposable technologies, which are simple to use and thus reduce the need for investment in expensive equipment and cleaning validation.