
An environmental study of single-use process technology for biopharmaceutical manufacturing offers a comprehensive examination of environmental impacts across the full process train using lifecycle assessment.
Vice President of marketing and disposables implementation at Biopharm Services. She is also the European chair of ISPE's Community of Practice for Disposable Technologies.

An environmental study of single-use process technology for biopharmaceutical manufacturing offers a comprehensive examination of environmental impacts across the full process train using lifecycle assessment.
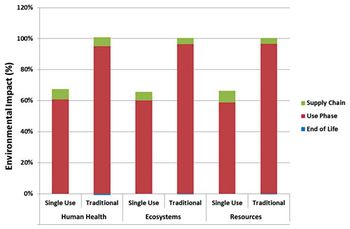
An environmental study of single-use process technology for biopharmaceutical manufacturing offers a comprehensive examination of environmental impacts across the full process train using lifecycle assessment.
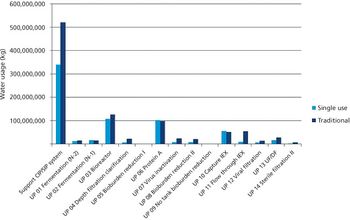
The authors compare the environmental impact of monoclonal antibody production using fixed-in-place processing and single-use systems.
Sanofi Pasteur's disposables implementation plan is part of a larger evaluation of technology innovation. Here's how they approach it.

How a Big Pharma company tackled the move to disposable bioreactors.

How this Big Pharma company successfully implemented disposable technologies in its manufacturing plant.

Suppliers, manufacturers, and governments must work together to plan how best to develop and deploy disposable systems for emergency response.

Single-use technologies can be configured and installed fairly quickly, but are they ready to handle the urgency and scale of a pandemic?

What end users think about single-use systems.
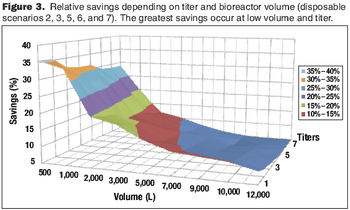
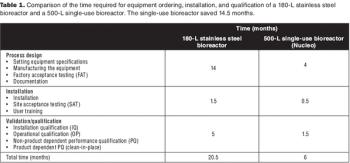
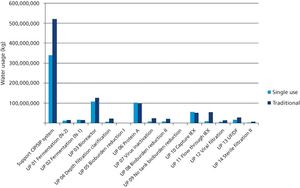
Cost modeling provides valuable insights to support strategic decision-making when implementing disposable technologies.

How to choose a disposable mixing system that fits your particular needs.
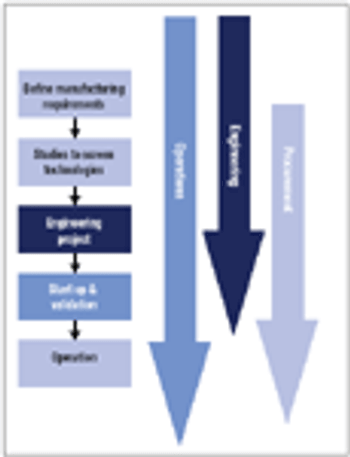
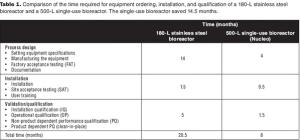
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.
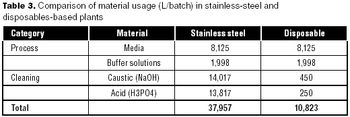
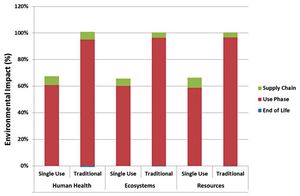
Can disposables reduce your facility's environmental footprint? We have compared the environmental footprint of a traditional biopharmaceutical manufacturing facility using fixed-in-place stainless steel equipment, and a facility implementing disposable technologies for cell culture, solution mixing and hold, product hold, and liquid transfer.
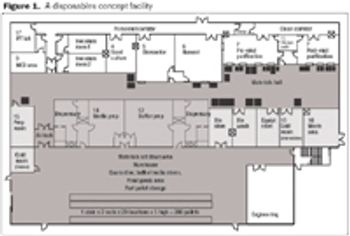
In addition to making technical developments, vendors are also looking at ways to improve supply-chain security. By offering standard, off-the-shelf products, vendors are able to shorten lead times and improve the security of supply.
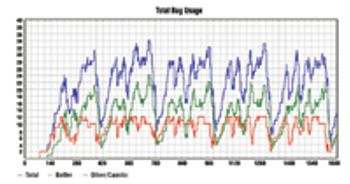
In its early days, the biotech industry was almost entirely science driven, but it has since expanded from a laboratory environment to a sophisticated and dynamic manufacturing environment. As technological discoveries are increasingly translated into commercial products, biotech companies are realizing that they must generate a stronger return on assets.
Published: March 1st 2014 | Updated:
Published: November 2nd 2011 | Updated:

Published: August 1st 2010 | Updated:

Published: February 1st 2010 | Updated:
Published: December 1st 2010 | Updated:

Published: December 1st 2009 | Updated: