
The wearable devices for the delivery of biologic products are now being manufactured and will be tested in clinical trials in the near future, according to the company.

The wearable devices for the delivery of biologic products are now being manufactured and will be tested in clinical trials in the near future, according to the company.

The collaboration will provide GMP manufacturing ahead of future clinical studies.

A new study in Nature Communications explores how to remove the bulk of the soaps that are added to injectables to make hydrophobic drugs more soluble.

The company’s method reduces the time required to crystallize antibodies from weeks to one day.

MilliporeSigma expands its Carlsbad, California-based GMP capacity for viral and gene-therapy products by nearly 90%.

The FlexPro 50, from Groninger, is a modular filling and closing system designed to process vials, cartridges, and syringes, as well as vials in bulk and trays.

Collaboration and single-use technologies aided the rapid scale-up of Ebola vaccine manufacturing

Selecting a delivery method early on may be beneficial.

Demand is driving expansion and consolidation of formulation and clinical trial materials services.

Headspace moisture analysis is a rapid non-destructive analytical method that may potentially address the limitations of traditional methods used for residual moisture determination.

The researchers examine the top 20 cancer drugs dosed by body size in the US, and estimate that drug companies will earn $1.8 billion in 2016 in revenue from leftover cancer drugs.

The US DEA will allow Kannalife access to pharmaceutical-grade cannabidiol to conduct feasibility studies for a potential treatment for neurodegenerative diseases.

A reformulated version of Rose Bengal, PV-10, may be used to treat melanoma when injected directly into tumors.
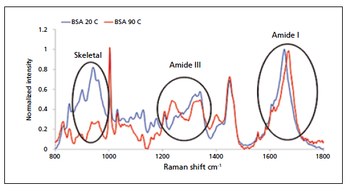
In this article, the author reviews some of the techniques that can yield valuable information on protein stability, focusing specifically on protein aggregation. Emphasis is placed on the enhanced information made available when technologies are used orthogonally, and the alignment of different approaches with specific stages of the biopharmaceutical development workflow.

Researchers from Oregon State University develop a new three-drug delivery system for cancer treatment.

The company plans to reformulate injectable products to make them into inhaled and intranasal medications.

Paras Biopharmaceuticals and Novozymes will collaborate on the creation of an improved osteoporosis treatment.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

A new consortium involving Arecor, FUJIFILM Diosynth Biotechnologies and the Center for Process Innovation will focus on formulation innovation as a way to improve downstream processing and reduce biopharmaceutical cost.
The global supply chain for bovine and porcine heparin and regulatory considerations are examined.

The company presented a portfolio of new products during the meeting in Madrid.

This article introduces the technology that powers automated HT–DLS and explores its practical applications in enhancing formulation stability investigations.

The project seeks to index the properties of various proteins in solution and create a home for data on the performance of product excipients with their active substances.

Although the Committee for Medicinal Products for Human Use (CHMP) gave Blincyto a positive opinion, full approval of the drug in the EMA will rely on additional clinical studies.