
The US Patent and Trademark Office issued three new patents that extend protection for Alexion’s rare-disease drug, Soliris, for an additional 10 years.

The US Patent and Trademark Office issued three new patents that extend protection for Alexion’s rare-disease drug, Soliris, for an additional 10 years.

Lonza further expands its micronization services with the acquisition of Swiss contract manufacturer, Micro-Macinazione, following its previous $5.5-billion acquisition of dosage form provider, Capsugel.

Media manufacturers are focused on reducing risk, improving quality and consistency, and managing costs.

Managing and prioritizing risk is essential to ensuring raw material quality. USP is developing new guidelines to make the work easier.

Catalent Applied Drug Delivery Institute announced a partnership with Rutgers University to examine the challenges of pediatric drug formulation and delivery.

The United Kingdom’s Center for Process Innovation (CPI) is investing in a new project, Microstar, which seeks to reduce risk for formulators through the development of accelerated screening methods for predictive design.

Microdermics will focus on product development and clinical activities of new drug delivery methods, while Vetter’s primary role will be in the fill and finish aspect.
The unique structures of fusion proteins lead to expression, heterogeneity, and stability issues.
OrlaSURF technology can be used for the development of target-binding assays to monitor the binding of an ADC to its antigen.
Including next-gen antibodies in pharma pipelines is considered essential for future success.

Communication and taking the time to develop the process are key to successful transfer and scale up of biologics

Primary packaging and container design reflects a move to patient-friendly formulations and delivery systems.

Researchers test the efficacy of a new polymer that is an alternative to PEG for drugs used to treat type 2 diabetes.

The baculovirus-insect cell system can produce large quantities of complex protein in a short period of time.

This report describes the first known attempt at quantifying the success of such processes in inducing nucleation on a 56–m2 freeze dryer operating at a load of 195,960 vials.

Avecia is adding drug substance capacity at its Milford, MA manufacturing site.
The development of mAb formulations poses challenges at the manufacturing, stability, analytical, and administration levels.

Interactions between biologic drug products and the components of prefilled syringes can cause protein aggregation, but there are alternative materials that can help mitigate this problem.

Prefilled syringes for commercial supply of the etanercept biosimilar will be produced at Catalent’s Brussels facility in Belgium.

High-purity low-endotoxin sugars improve robustness and stability of protein formulation and improve drug product quality.

Multiparticulates are increasingly used due to their flexibility in providing controlled-release, fixed-dose combinations, ease of taste-masking, and suitability for pediatric applications

Challenging molecules and markets are driving the development of new solutions for drug delivery.
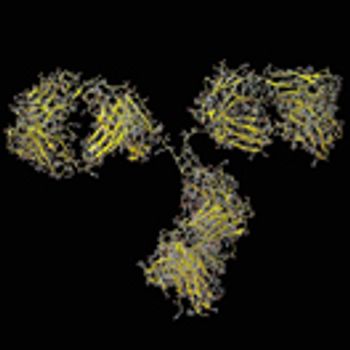
An approach to stabilize PBS-based formulations could provide a simple physiological solution for use of proteins in research, preclinical, diagnostics, and clinical studies, as well as commercial biotherapeutic products.

Amgen discussed the drug-delivery approaches to two of its biologics in a recent second-quarter earnings call.

NIST’s monoclonal antibody reference material can be used as a standard for biopharmaceutical analytical quality control.