
The companies have entered into a global licensing and collaboration agreement to commercialize ReForm excipients used in biotherapeutic formulations.

The companies have entered into a global licensing and collaboration agreement to commercialize ReForm excipients used in biotherapeutic formulations.

Biologics raise unique formulation and development challenges, and industry is still on a learning curve to get the best out of these diverse and complex therapies.
This study aims to use plant-leaf extract for the green synthesis of gold nanoparticles and to evaluate their antibacterial and antioxidant activity.

The new Technology Excellence Center, located in Boston, MA, will further the company’s presence in the United States biopharma drug development landscape.

BASF launches digital solutions for the pharma industry during CPhI Worldwide 2019.

The companies will develop innovative formulations for drugs to treat rare diseases using Catalent’s oral disintegrating tablet technologies.

CHMP has recommended that Ervebo (rVSVΔG-ZEBOV-GP), a vaccine for active immunization against Ebola, be granted conditional marketing authorization in the EU.

On Tuesday, Nov. 5, 2019, Karima Yadi from Becton Dickinson & Co. will present the challenges of using, and necessity for, shorter needles and integrated systems with high viscosity drugs, such as biologics, to improve the patient experience at CPhI Worldwide.

Leveraging automation and a step-by-step approach are keys to success.

FDA has approved Novo Nordisk’s Rybelsus (semaglutide), a glucagon-like peptide receptor protein treatment in oral tablet form for type 2 diabetes.

Innovation in manufacturing technologies must occur to ensure the availability of gene and cell therapies.

The evolution of cell-culture technology is driving the need for improvements in modeling solutions.
Research advances have enabled the application of nanotechnology to drug delivery. What does this technology offer in the way of enhancing therapeutic effect?

Nanobiotix has launched Curadigm, a spinoff company that will specialize in developing a nanotechnology platform for healthcare applications.

Intertek has announced the expansion of its pharmaceutical services laboratory in Melbourn, near Cambridge, UK, through the acquisition of a new 20,000 sq. ft facility.

Sterile filtration is often required for biologics but presents degradation and compatibility challenges.

As biologics continue to push boundaries, the industry needs to take a holistic approach to formulation to ensure success.

Jessica Rousset, chief operating officer, CURE Pharmaceutical, highlights some key regulatory considerations developers should keep in mind when approaching alternative dosage forms.

The technology uses a silica nanoparticle to deliver vaccines and cancer treatments.

Optimizing the patient experience and technological advances can positively impact adherence.

The drug is approved in the United States specifically for treating acquired thrombotic thrombocytopenic purpura, a rare blood-clotting disorder.

To ensure the sterility of parenteral biopharmaceutical products, it is necessary to employ certain tools, technologies, and standard operating procedures.

Formulation issues cause significant drug project delays and project failures, according to a 2018 survey conducted by Informa Pharma Intelligence, Rentschler Biopharma, and LEUKOCARE.
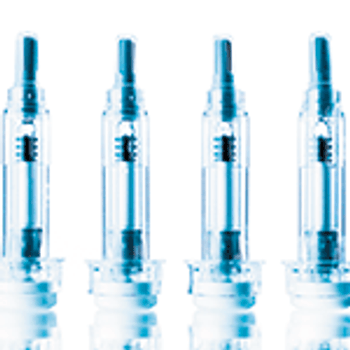
As biologic drugs grow increasingly complex, drug delivery mechanisms such as prefilled syringes are being adapted to meet the challenges.

Industry experts discuss the formulation and development issues that should be considered when addressing scale up from small-scale batches to commercial production.