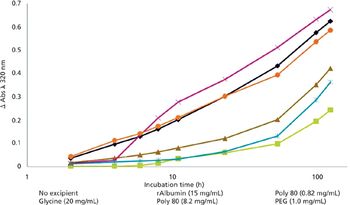
Recombinant albumin can stabilize a drug product and assist in API release.

Recombinant albumin can stabilize a drug product and assist in API release.
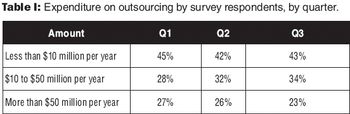
A survey provides insight into drug companies' plans for spending on outsourced services. This article contains bonus online material.


Vetter held a groundbreaking ceremony for its new facility in Ravensburg.
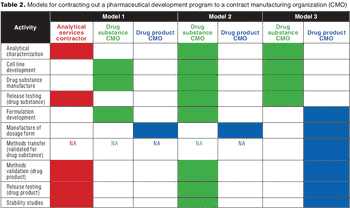
Formulation strategy is an important consideration when selecting and managing outsourced biopharmaceutical development programs.
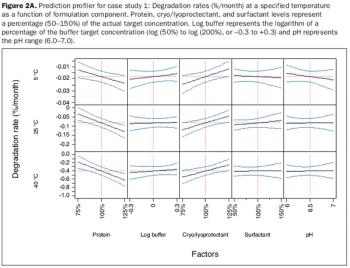
Design of experiments is a valuable tool for identifying aspects of a formulation that are critical to product quality.
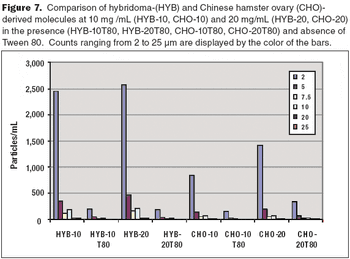
How to maintain product stability and prevent particulates.
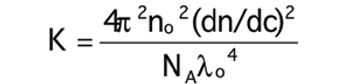
Can increase in ionic strength result in higher viscosity?
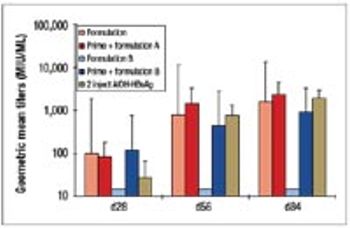
The disadvantages of the traditional vaccine regime (prime plus boost) have spurred the development of single-shot vaccines. This article describes the development and manufacture of a prototype single-shot vaccine that uses microspheres made from cross-linked modified dextran polymers for controlled release of the antigen.
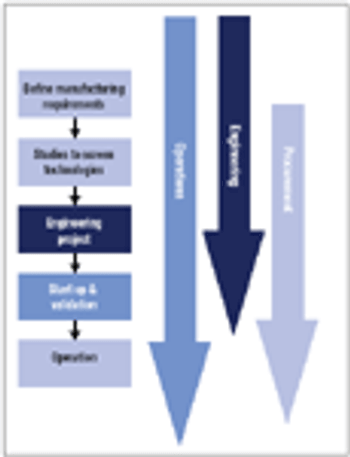
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.

The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.
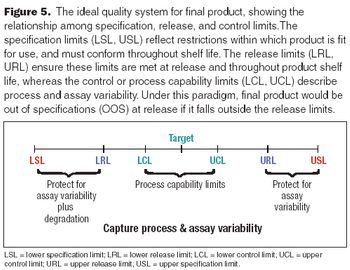
Set limits to provide incentives for process improvements.
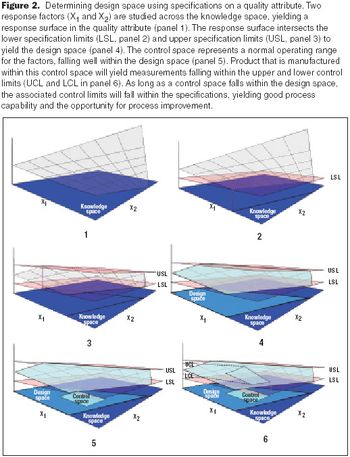
Resolve confusion about measurements.

US Food and Drug Administration's Division of Biologic Oncology Products has approved two new biologics license application (BLA) supplements expanding the approval of Genentech's Herceptin (trastuzumab) for the treatment of breast cancer.
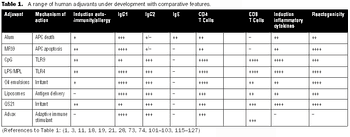
Adjuvant-caused vaccine reactions are one of the most important barriers to better acceptance of routine prophylactic vaccination.

The biopharmaceutical industry has gained a lot of experience in monitoring glycosylation, but still has a lot to learn about the structure–function relationship.

The transdermal delivery of biologics-as well as of conventional drugs-is growing in popularity because the technique offers numerous advantages.

The type of reactive moiety controls the site and stability of the covalent link and also the total number of PEGylation sites on a given protein.