
Biogen plans to build a biologics manufacturing plant in northwest Switzerland using next-generation technologies to create efficiency and sustainability.

Biogen plans to build a biologics manufacturing plant in northwest Switzerland using next-generation technologies to create efficiency and sustainability.

The new facility expands the company’s commercial manufacturing capability at its Bend, Ore. site.

Australian company, Genea Biocells, will open a facility in San Diego, California for cell and culture-media manufacturing and R&D.

Lonza’s planned facility will be used to develop and manufacture viral gene therapies and virally modified cell therapies
Single-use and modular technologies plus continuous manufacturing are increasingly important to biopharma scale-up and tech transfer.

The costs and benefits of integrating modular concepts for on-demand bioprocessing are explored.

Prequalified manufacturing suites could benefit from a new business model, say some industry executives.

Facilities in China, Ireland, Germany, and the United States have been recognized by ISPE in the 2015 Facility of the Year Awards program.

The upgrades will offer the opportunity for higher product yields and higher purity levels.

Baxter announced that its new biopharmaceutical arm, scheduled to become independent from Baxter in 2015, will be located in Bannockburn, Illinois.

The multi-product biopharmaceutical manufacturing facility is scheduled to start up in 2017.

The new location will increase Bristol-Myers Squibb's biologics manufacturing capacity.

Sartorius opened a new application center at its offices in Shanghai for product demonstrations, trial runs, and training sessions.

The renovation to Roche's historic office building in Basel, Switzerland will feature sustainable workplaces and a state-of-the-art research center.
Experts discuss the future of modular manufacturing and the challenges that biopharma manufacturers face in facility design

Walker Barrier Systems builds eight mobile clean rooms for the Texas A&M Center for Innovation in Advanced Development and Manufacturing.

GE Healthcare Life Sciences announced that it will build a KUBio modular biopharmaceutical factory in China for JHL Biotech.

BioPharm International spoke with INTERPHEX 2013 conference-session presenters to gain insight on trends in facility and process design.

Additional challenges to the new cleanroom paradigm from concurrent multiproduct manufacturing of bulk drug substances in a controlled non-classified (CNC) ballroom environment.
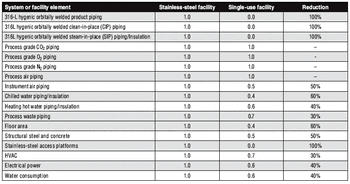
The move to single-use manufacturing has prompted a paradigm shift in facility design.

NIBRT's Michael Lacey provides an overview of biopharmaceutical facility design and operation.

This article describes best practices for implementing a single-use process train at a bioproduction facility.
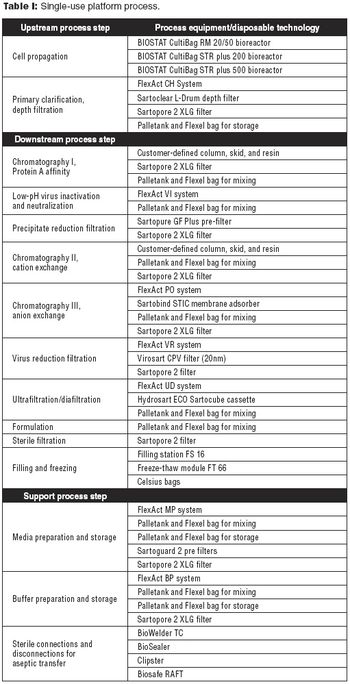
The combination of single-use platform technology with modular facility construction is a template for flexible manufacturing.

A brief case study of a facility-fit analysis provides insight into how to adjust capacity when moving from clinical-to commercial-scale production.

The authors explain why Catalent decided to transition from stainless steel to single-use systems.