
Single-use technology is gaining ground in downstream bioprocessing, but challenges stall further adoption.

Single-use technology is gaining ground in downstream bioprocessing, but challenges stall further adoption.

This article takes a look at current practices for cleaning and sterilizing biomanufacturing equipment used in a multi-product versus single-product setting.

The Quartic Platform is a new, AI-powered smart manufacturing platform that can be integrated into legacy facilities and manufacturing processes.

Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.

In a keynote session at INTERPHEX 2019, experts will review and debate the issues and present potential solutions for contamination issues in aseptic manufacturing.

3D printing offers a new design freedom for bio/pharmaceutical manufacturing: whether for “printing” a solid-dosage drug or for creating a piece of equipment for bio/pharmaceutical laboratories or manufacturing facilities.

Getting the science right helps biopharma startups overcome development and commercialization challenges.

A look at the skill sets and training needed to tackle the increasing levels of automation in bioprocessing facilities.

The acquisition of the site in Copenhagen, Denmark, will significantly expand Fujifilm’s capacity and capabilities.

The new facility, located at the company’s headquarters in Pittsburgh, PA, is expected to meet all clinical and commercial development needs of the company’s lead gene therapy program.

A properly designed validation program will detect variation and ensure control based on process risk.
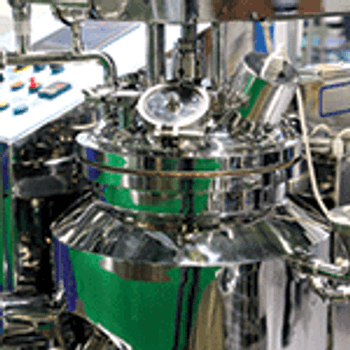
A look at recent investments by contract manufacturers to increase single-use bioreactor capacity.

The company will invest an additional $60 million to expand its manufacturing facility in Durham County, NC.

Suppliers address the complexity of supplying disposable components for single-use systems to the global biopharmaceutical manufacturing industry.

Single-use components for biopharmaceutical manufacturing have a lower environmental impact than reusable components, but disposal is still a consideration.

G-CON will provide a complete cleanroom infrastructure for GE Healthcare’s cell therapy and viral vector production platforms that will simplify early-stage manufacturing efforts.
For rapid scale-up of biomanufacturing under expedited review status, facility design must better integrate product development and manufacturing lifecycle activities.

The company is investing approximately $14 million to expand biologics packaging capabilities and capacity at its biologics manufacturing facility in Bloomington, IN.

The company will use GE Healthcare’s off-the-shelf KUBio biologics factory, which is expected to start operations in 2020, to provide development and manufacturing for early- to late-clinical and early-commercial manufacturing stages.

FDA’s 21st Century goals can be realized by using a multi-purpose manufacturing facility with a flexible design that provides reliable production without extensive regulatory oversight.

A multi-purpose biopharmaceutical manufacturing facility using a matrix of multi-functional cleanrooms can be adapted to efficiently meet the capacity challenges of both supplying clinical trials and launching products.

A matrix of multi-functional cleanrooms can be adapted for launching products.

The new center will integrate biologics drug discovery, development, clinical manufacturing, and commercial manufacturing.

The vaccine producer announced an expansion to its Holly Springs, NC, manufacturing facility where it will increase production of its cell-based quadrivalent influenza vaccine.

The new collaborative center aims to serve as a hub for innovations in drug development and manufacturing.