
Genentech’s biologics drug substance plant is the overall winner of the International Society for Pharmaceutical Engineering’s 2016 FOYA Awards.

Genentech’s biologics drug substance plant is the overall winner of the International Society for Pharmaceutical Engineering’s 2016 FOYA Awards.

Clinical biotechnology company Moderna Therapeutics will build an integrated clinical manufacturing facility for mRNA production in Norwood, Massachusetts.

GE Healthcare’s GE BioPark Cork will hold four KUBio manufacturing facilities; GE will also collaborate with NIBRT for biopharmaceutical training.

Susan Schniepp, Distinguished Fellow at Regulatory Compliance Associates, discusses the regulatory requirements for improving manufacturing lines.
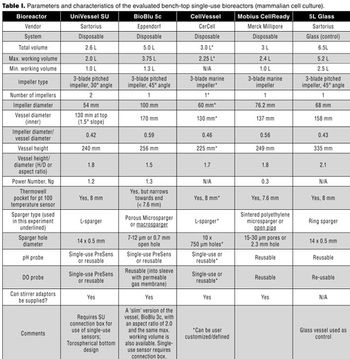
The authors present a case study in which four single-use vessels were fitted to an existing bioreactor system.

Novo Nordisk broke ground on an expanded production plant for insulin in Kalundborg, Denmark.

Revised versions of ISO 14644 Parts 1 and 2 introduce changes to sampling procedures and monitoring plans for cleanrooms and clean zones.

A streamlined approach may enhance process efficiency and product quality.

Pfizer broke ground at its Andover, Massachusetts campus on a clinical manufacturing facility for complex biologics and vaccines.

Alvotech prepares for commercial biosimilar production in new facility with single-use bioreactors in Reykjavik, Iceland.

MilliporeSigma, the North American life-sciences business of Germany’s Merck KGaA, added a 2000-L single-use bioreactor to its facility in Massachusetts.

EMD Serono, the North American biopharmaceutical business of Germany’s Merck KGaA, will expand its R&D facility in Massachusetts, US.

Shire announces plans to build a flexible biopharmaceutical manufacturing facility in County Meath, Ireland.

A team of Bristol-Myers Squibb scientists will work in a new laboratory at the National Institute of Bioprocessing Research and Training facility in Dublin, Ireland.

Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.

TxCell’s new facilities are based in Sophia Antipolis, France, on the premises of GenBiotech.
Asking the right questions is crucial to establishing a biopharmaceutical facility design.

The International Society for Pharmaceutical Engineering (ISPE) Facility of the Year Awards (FOYA) program announced its 2016 Category Award winners for operational excellence, sustainability, process innovation, project execution, equipment innovation, and facility integration.

Novasep is building a new synthesis laboratory and adding capacity for kilogram-scale batches of synthetic molecules that are needed for biological testing and preclinical trials, at its Pennsylvania, US facility.

Samsung BioLogics begins construction on their third facility in Songdo, Korea.

Biogen, Genentech, Johnson & Johnson, Novartis, and Patheon publicize their support for action on climate change.

GE Healthcare's KUBio prebuilt modules were shipped from Germany to JHL Biotech in Wuhan, China.

Hiroaki Suzuki will lead Vetter’s business activities in the new Tokyo-based office.

The National Biologics Manufacturing Centre will provide companies with open access to bioprocessing facilities and expertise to expedite low-risk market entry of complex biologics.

Preventing contamination requires quality systems to be in place, including routine cleaning, a robust cleaning validation program, and preventive maintenance.