
Using a competency-based approach to effectively train biopharmaceutical industry staff.

Using a competency-based approach to effectively train biopharmaceutical industry staff.
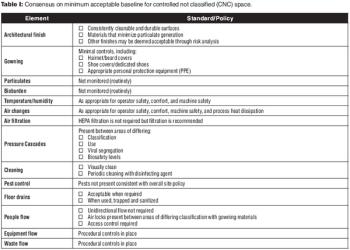
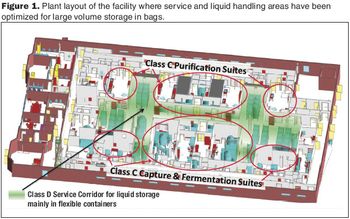
The authors re-examine environmental controls in the context of technical advances in manufacturing.
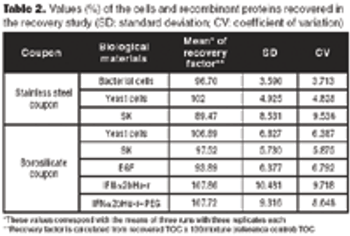
Case studies show TOC is effective for cleaning validation.
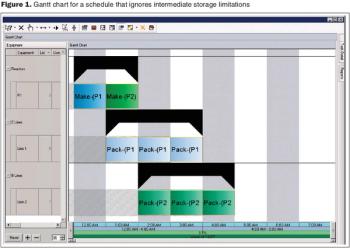
An algorithmic approach to fine tune facility design and predict capacity.
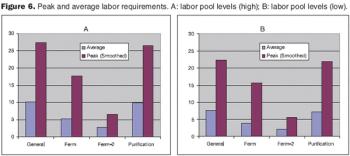
How to improve facility design and predict capacity.
A case study compares capital costs, operating expenses, and NPV for a new MAb plant.

To assess current trends in cleanrooms and engineering & facilities, BioPharm International turned to Parrish Galliher, founder and chief technology officer, Xcellerex, Inc.; Jim Maslowski, owner, PDC Aseptic Filling Systems; Morgan Polen, vice president, application technology, Lighthouse Worldwide Solutions; and Benoît Verjans, commercial director, Aseptic Technologies.

A close-up look at Pfizer's biotherapeutics plant in Shanbally, Ireland.

The development of a skilled labor force is essential for an expanding biopharmaceutical industry.
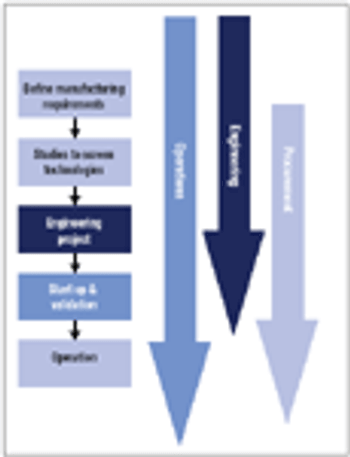
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.
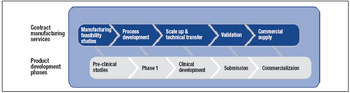
Four reasons why outsourcing may be the best option, and key factors to consider when selecting a provider.

There could be a serious glut of commercial scale mammalian cell culture capacity over the next five years. Then again, there could be a significant shortage. It all depends on how things develop in expression technology, the new product pipeline, and corporate strategies.

When making critical decisions such as whether to build or buy critical capabilities, companies need a decision-making approach that weighs risks and rewards as a science with adequate inputs, repeatable processes, and measurable results. The method must also accommodate the human factor by encouraging wide participation and providing the kind of neutral decision criteria that satisfies participants about the objectivity of the process.

Manufacturers of biopharmaceuticals can improve productivity by taking patient wellness into account.
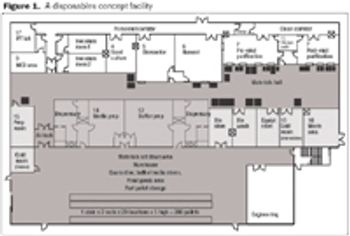
In addition to making technical developments, vendors are also looking at ways to improve supply-chain security. By offering standard, off-the-shelf products, vendors are able to shorten lead times and improve the security of supply.

Bayer Schering Pharma AG, (Berlin, Germany) has completed the acquisition of a biologics manufacturing facility in Emeryville, CA, from Novartis.

Discovery Laboratories (Warrington, PA) could see the end of its struggle to launch its Surfaxin (lucinactant) drug on the US market soon, as the manufacturing issues it has faced have been resolved.

PAREXEL Consulting (Boston, MA) has hired three senior GMP consultants based in Europe with the hope of helping its clients address complexities of European Union directives in areas such as manufacturing regulations, quality, and safety.

SAFC (St. Louis, MO), a member of the Sigma-Aldrich Group, has announced a $29-million investment to significantly expand its drug substance capabilities in high-potency biologics at the Sigma-Aldrich facility in Jerusalem, Israel

Bristol-Myers Squibb (Princeton, NJ) will acquire privately held Adnexus Therapeutics (Waltham, MA), the developer of a new therapeutic class of biologics called Adnectins.

Favrille, a San Diego-based biopharmaceutical company, is one of a handful of firms on the forefront of personalized medicine. Because personalized treatment is tailored to an individual's biology, it has the potential to be far more effective than current approaches to disease management.


From the earliest days of the biotechnology industry, companies have grappled with the complexities of making innovative biopharmaceuticals on a large scale. Success in manufacturing begins with process science, since biotech production requires perfection in maintaining living organisms in a sterile environment under controlled physiological conditions. But unless companies can solve the challenge of planning for and managing manufacturing capacity, they will not be able to achieve the full potential of promising biotech products.

The mounting threats of pandemic influenza, bioterrorism, and emerging infectious diseases continue to be the focus of research programs and funding initiatives, not only within governmental agencies, but also universities, private research firms, and commercial manufacturing entities. With all of these efforts, however, the question of manufacturing capacity and the ability to respond to pandemic and emerging threats continues to be a major concern.

Roche (Basel, Switzerland, www.roche.com) has opened its new biotechnology production center in Basel.