
The companies have established a joint laboratory to develop full continuous processing to manufacture high yields of monoclonal antibodies at reduced costs.

The companies have established a joint laboratory to develop full continuous processing to manufacture high yields of monoclonal antibodies at reduced costs.

The API manufacturer has announced that it has completed the expansion of large-scale manufacturing capabilities at its Charles City, Iowa site.

The partnership and the formation of the institute intend to bring together industry, academia, and regulators to tackle challenges and provide solutions for continuous manufacturing.

The company is investing EUR 170 million (US$201 million) to expand its vaccine manufacturing site in France for its newest influenza vaccine.

Johnson & Johnson’s Janssen Sciences Ireland UC will invest more than EUR 300 million (US$355 million) to expand manufacturing capacity for biologic medicines at its Ireland facility.

Sartorius begins building a new, EUR 30-million (US$35.2 million) Cell Culture Technology Center in Ulm, Germany.

Eurofins Scientific has opened a £4-million (US$5-million) pharmaceutical chemistry and microbiology facility in Livingston, Scotland, to support the company’s product and water testing businesses in the UK.

Fresenius Kabi broke ground on a previously announced $250 million expansion of its Melrose Park, IL, manufacturing facility.

Modeling tools help process engineers optimize a biopharmaceutical facility’s capacity.

The International Society for Pharmaceutical Engineering announced the release of ISPE GAMP Good Practice Guide: IT Infrastructure Control and Compliance (Second Edition).

FDA noted in a recent inspection that US Stem Cell Clinic was processing and administering stem cell treatments that were neither reviewed nor approved by the agency.

The generic pharmaceuticals firm has sold its Baddi, India formulations manufacturing facility following a recent fire at its joint-venture plant in Algeria.

Laboratory tests can determine critical cleaning parameters for passivation treatments used to prevent rouge on GMP stainless-steel equipment.

Roquette, a biopharmaceutical company specializing in plant-based excipients, announced the opening of a new R&D and customer technical service facility in Singapore in fall 2017.

GlaxoSmithKline (GSK) opened a new vaccine manufacturing facility in Montrose, Scotland. The £44 million (US$57 million) facility will be used to manufacture aluminum salts, which are used for vaccine production.

The acquisition adds sterile manufacturing capability to the CDMO’s services, which include API formulation and manufacturing.

The Washington-headquartered biotech firm plans to use the newly purchased facility to produce antibodies for its current and future pipeline.

Lonza introduces new modular complex that offers flexibility and individually tailored solutions to biomanufacturing challenges.

The company has broken ground on a R&D and process development facility in Missouri.

Improving the bioreactor growth environment increases the rigor of bioprocessing runs.
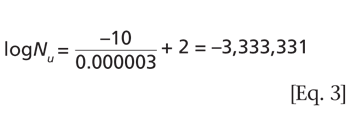
Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

The Basel office will house the clinical development team and other functions to progress the company’s lead compound emapalumab.

The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.

In a press release, Momenta announced on Feb. 16, 2017 that Momenta/Sandoz’s fill/finish contract manufacturer, Pfizer, received a warning letter for the manufacture of the 40 mg of Glatopa (glatiramer acetate injection), Momenta’s generic version of the drug Copaxone. Pfizer said in the statement that the warning letter does not affect the production or shipment of the 20 mg version of the drug, which is already approved by FDA.

A Q&A with PCI Pharma Services about best practices for choosing and maintaining temperature sensors in cold and cryogenic storage.