
Pressures to reduce healthcare spending generates proposals to spur competition, cut costs.

Pressures to reduce healthcare spending generates proposals to spur competition, cut costs.

A culture of quality that emphasizes business objectives, risk management, and the informed application of technology can improve compliance.
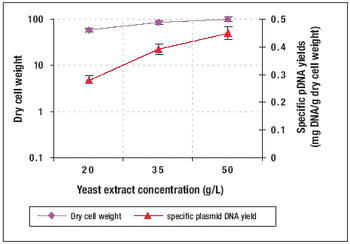
How to produce Plasmid DNA in a high-cell-density culture.

The FDA seeks new strategies for improving the safe use of opioids and other high-risk medicines, including erythropoiesis stimulating agents.

Agency officials promise swift action against violators of drug safety and quality regulations.
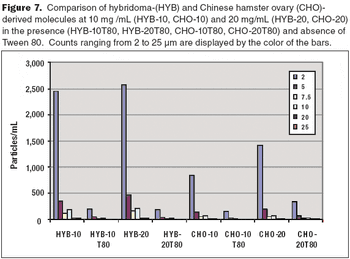
How to maintain product stability and prevent particulates.

New expression systems compete for attention.

Use Lean techniques to improve manufacturing compliance

Without a rigorous discussion of the pros and cons of QbD, its tremendous benefits will be lost.

Authorities are pushing for CE; manufacturers prefer to focus on value.

The FDA is poised to gain more authority and resources to ensure product quality.
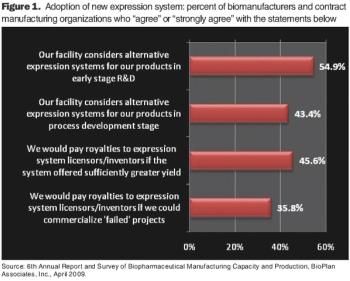
With all of the new expressions systems being developed, companies must decide what improved production and yield are really worth.
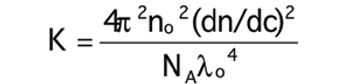
Can increase in ionic strength result in higher viscosity?

The 45 comments submitted raised concerns about legacy products and ongoing process monitoring.
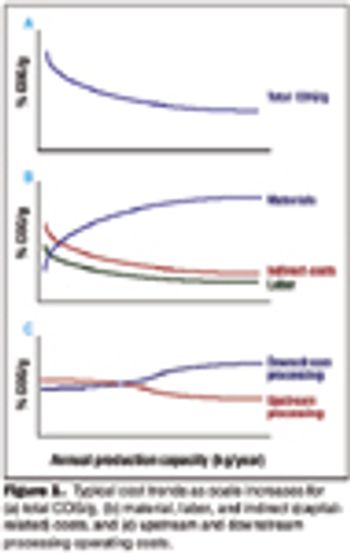
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

Altering the order of operations, using new resins, and increasing dynamic binding capacity can obviate the need for major facilty changes.

A purification scheme to maximize the efficiency of the purification process and product purity while minimizing the development time for early-phase therapeutic antibodies.
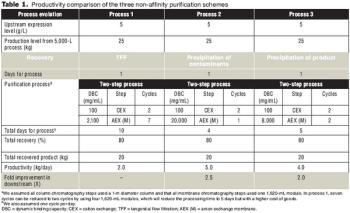
In three non-affinity purification processes based on cation exchange capture with high binding capacity, applying a host cell protein exclusion strategy enabled robust scale up and better economics.
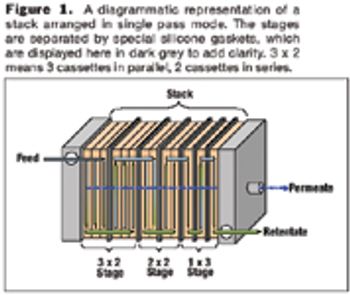
New techniques can greatly improve the MAb purification process.
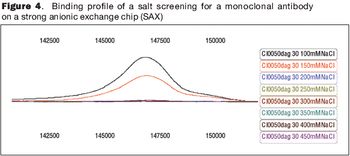
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.
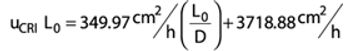
Robust packing procedures can improve process performance and increase resin lifetime.

A close-up look at Pfizer's biotherapeutics plant in Shanbally, Ireland.
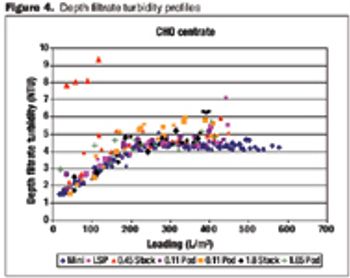
Data on the performance and variability of different formats.

What will it take for the industry to embrace new hosts?

Broader transparency in product prices and payments to researchers aim to curb conflicts of interest and rationalize drug expenditures.