
For cellular therapies to become a viable treatment for large-sized patient groups, steps need to be taken to develop an efficient manufacturing and supply system to minimise the cost of goods.

For cellular therapies to become a viable treatment for large-sized patient groups, steps need to be taken to develop an efficient manufacturing and supply system to minimise the cost of goods.

New research will focus on a superfamily of protein receptors linked to various diseases.

The agency recommends that companies developing drugs to treat Ebola apply for orphan drug designation.

The new service offering will help customers determine levels of permeability, transport, metabolism, and toxicity in drug product.

Personalized immunotherapy treatments help 90% of acute lymphoblastic leukemia patients achieve remission in a new study.
Prefilled syringes offer advantages to manufacturers, healthcare professionals, and patients.

NIH continues funding for tissue chips to be used in the development of therapeutics.

GS1 publishes a healthcare industry guideline describing how to implement GS1 standards to support requirements of the 2013 US Drug Supply Chain Security Act.

The BioPhorum Operations Group has published a resource to help the biopharmaceutical industry deliver a consistent approach to continued process verification.

FDA cites cGMP violations for API manufacturing at a facility in Tianjin, China.

FDA inspection found cGMP violations in the Bangalore, India API manufacturing facility.

Experts give insight on method transfer, QbD, and regulations for analytical method development and validation for biopharmaceuticals.

FDA finalizes guidance on expedited programs for new drug approvals for treatment of serious and life-threatening conditions.

Merck KGaA fill-finish expansion in Italy will be completed in 2017.

The Pediatric Clinical Research Group initiative expands to include pediatric research sites.

New approaches to vaccine production are targeting rapid supply for pandemic situations and broadly effective therapeutic treatments.
The presence of minute amounts of chelators can help minimize the degradation of monoclonal antibodies.

As Europe strives to firmly incorporate quality-by-design principles, there are several key issues that still need to be addressed.

HHS plan makes progress in ensuring availability of safe vaccines.
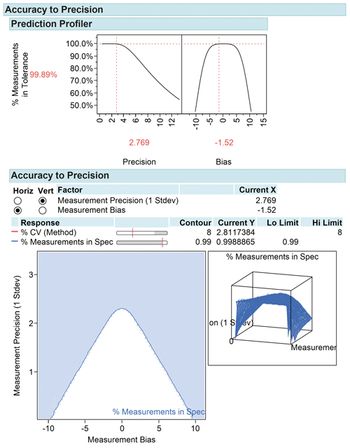
Design of experiment is a powerful development tool for method characterization and method validation.
The targeted delivery of cytotoxic drugs using antibody drug conjugates would not be possible without effective linkers to connect and then release the key chemical and biological materials.

Thirteen companies are accepted for participation in the supply chain program.

NCI launches trial to assess the utility of genetic sequencing to improve patient outcomes.

Genmab enters collaboration with Eli Lilly to use and evaluate Genmab's DuoBody technology for bispecific antibodies.
Antibody fragments pose unique challenges in recovery, purification, and formulation.