
USP Hosts Symposium on Science and Standards

USP Hosts Symposium on Science and Standards

The authors demonstrate how an integrated model is helping to achieve regulatory flexibility. This article is part of a special section on biopharmaceutical trends.

Manufacturers and regulators struggle to control phony versions of crucial medicines.

Import controls and risk strategies aim to promote quality and spur new drug development.

A team from Northwestern University has demonstrated the feasibility of topical delivery of small interfering RNA (siRNA).
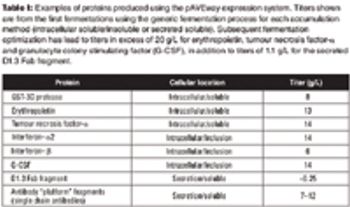
Industry experts discuss methods for optimizing protein expression in bacterial and mammalian cell lines.

Multiple initiatives are moving forward to maintain US leadership in biopharm R&D.
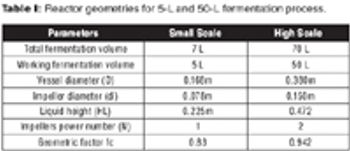
The authors present scale-up from a 5-L fermentor to a 50-L pilot-scale using the criterion of constant power consumption per unit liquid volume.

In a special anniversary interview, Washington Editor Jill Wechsler speaks with with FDA Deputy Commissioner Deborah Autor about where the agency is headed.

Panayiotis P. Constantinides of Biopharmaceutical & Drug Delivery Consulting on growth of nanoparticle delivery systems.

Charles H. Squires of Pfenex discusses advances in expression platform solutions.

This article discusses potential opportunities to improve the patient experience through formulation and delivery device technologies.

Manufacturers seek clear path to develop safe-use approaches for more risky OTC therapies.

A Q&A with Alan Shaw of Vaxinnate. This article is part of a special section on expression systems.
Industry experts discuss the benefits and challenges of self-administration of injectable therapies.

A technical rountable featuring Sartorius Stedim Biotech, Pall Life Sciences, 3M Purification, Asahi Kasei Bioprocess, and Bio-Rad Laboratories.

Soaring opioid use creates challenges for new drug development and supply-chain control.
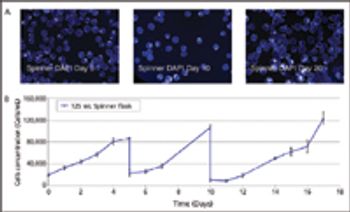
Demonstration of large-scale stem-cell scale-up.
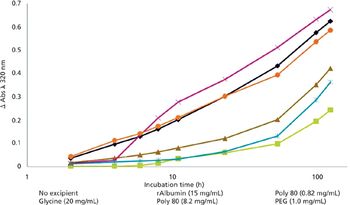
Recombinant albumin can stabilize a drug product and assist in API release.

Social media use raises questions about applying old standards to new information technology.

More collaboration and expanded oversight aim to compel manufacturers to follow GMPs.

Contract organizations must have highly organized teams and plans to accommodate today's audits.

Pressure to approve new user fees opens the door to action on drug shortages, prices, and regulation.

How does working in a virtual biotech environment affect biosimilar development? Anjan Selz outlines target and partner selection in this first of a two part article.
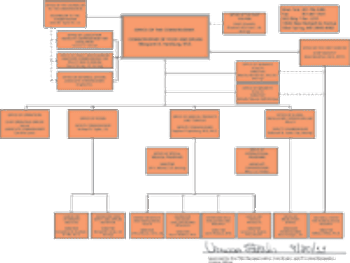
Added responsibilities and outside concerns prompt overhaul of agency's structure.